Respiratory infection is the most common human adenovirus (HAdV) disease accounting for 7–8% of viral respiratory diseases in children less than 5 years. Differentiation of bacterial infections and viral infections is a common clinical problem.
Material and methodsOne hundred oropharyngeal swabs obtained from October 2019 to November 2020 from patients attending the paediatric emergency room with suspicion of upper respiratory tract infection and negative results in influenza and RSV tests were included. Oropharyngeal swabs specimens were rapidly processed with STANDARD™ F Adeno Respi Ag FIA and the results were confirmed by RealStar® Adenovirus PCR Kit 1.0 (Altona diagnostics).
ResultsSTANDARD™ F Adeno Respi Ag FIA had sensitivity and specificity values of 71.93% and 100% respectively. The performance of the test was higher in samples from children younger than 24 months and taken less than 72h since the beginning of symptoms. In this subgroup the test had 88.8% sensitivity and 100% specificity.
ConclusionSTANDARD™ F Adeno Respi Ag FIA may improve the management of respiratory diseases in children younger than 24 months and less than 72h since the beginning of symptoms in paediatric emergency rooms.
Las infecciones respiratorias son la enfermedad más común asociada a los adenovirus humanos (AdvH)y causan del 7 al 8% de las enfermedades respiratorias víricas en niños menores de 5 años. La distinción entre las infecciones bacterianas y las víricas constituye un problema clínico frecuente.
Materiales y métodosEl estudio incluyó 100 hisopos orofaríngeos obtenidos entre octubre de 2019 y noviembre de 2020 de pacientes que habían acudido a los servicios de urgencias pediátricas con sospecha de infección de las vías respiratorias superiores y resultados negativos en las pruebas de gripe y VRS. Las muestras de los hisopos orofaríngeos se procesaron rápidamente con Standard™ F Adeno Respi Ag FIA y los resultados se confirmaron mediante RealStar® Adenovirus PCR Kit 1.0 (altona Diagnostics).
ResultadosStandard™ F Adeno Respi Ag FIA tenía unos valores de sensibilidad y especificidad del 71,93% y el 100%, respectivamente. El rendimiento de la prueba fue superior en muestras de niños menores de 24 meses y tomadas menos de 72 horas después del inicio de los síntomas. En este subgrupo, la prueba tuvo una sensibilidad del 88,8% y una especificidad del 100%.
ConclusiónStandard™ F Adeno Respi Ag FIA puede mejorar la gestión de las enfermedades respiratorias en niños menores de 24 meses con menos de 72 desde el inicio de los síntomas en servicios de urgencias pediátricas.
Human adenoviruses (HAdV) are non-enveloped, double-stranded DNA viruses that were first isolated in 1953 from human adenoid tissue.1 They belong to the Mastadenovirus genus and are currently grouped into nine subgroups (A to I) that comprise more than 100 genotypes.2,3 HAdV infection can be asymptomatic or result in a broad spectrum of clinical diseases, including gastrointestinal infections, keratoconjunctivitis, upper respiratory tract infections (pharyngitis, tonsillitis), pneumonia, myocarditis, hepatitis, haemorrhagic cystitis and meningitis.4 The HAdV types most commonly found in respiratory samples belong to HAdV species C (HAdV-C1, -C2, -C5, and -C6) and HAdV species B, subspecies B1 (HAdV-B3 and -B7) and B2 (HAdV-B14), which are endemic and epidemic respectively in paediatric populations.5 Respiratory infection is the most common disease caused by HAdV, accounting for 7–8% of viral respiratory diseases in children under 5 years.4 The incidence of HAdV infection peaks in infants and children occur between 6 months and 5 years of age.
Upper respiratory tract infections are a frequent reason for visits to primary care clinics and paediatric emergency rooms and can be caused by several viruses and bacteria, including human Coronavirus, human Adenovirus, human Metapneumovirus, human Bocavirus, Streptococcus pyogenes and others.6 Accurate diagnostic at the species level is important for patient management, particularly in pharyngitis cases, in which the major etiologies include viruses and S. pyogenes that have a very different therapeutic approach. Clinical and analytical findings may be consistent with both, HAdV and S. pyogenes infections, and for this reason tools that can identify viruses may result in less antibiotic pressure and shorter hospital stay.4 These advantages represent a better care of the patient, better use of antibiotics and less unnecessary complementary tests (blood tests, imaging etc.). This translates into lower costs for health care systems.
Our main objective was to evaluate a rapid test of HAdV antigen detection in oropharyngeal swabs negative for influenza and RSV, establishing sensitivity and specificity in this population. As side objectives were reviewed clinical manifestations and analytical findings in order to identify those situations in which the test has a better performance and would be more useful.
Patients and methodsOne hundred oropharyngeal swabs received in the Microbiology Department from October 2019 to November 2020 (most of the samples were collected in the periods from October 2019 to April 2020 and from October to November 2020), from patients that visited the paediatric emergency room with suspicion of upper respiratory tract infections and had negative results in Flu and RSV PCR assays, were included. All the determinations were done in the Microbiology Department by qualified staff. Samples were collected by the nursing staff following the sample collection protocols of the hospital and sent to the Microbiology Department following the local transport protocols for biological samples.
Oropharyngeal swabs specimens were rapidly processed with STANDARD™ F Adeno Respi Ag FIA according to the manufacturer's instructions. If HAdV is present in the sample, the adenoviruses combined with a monoclonal anti-HAdV are captured on the test line and produce a fluorescence signal. This is measured using the fluorescence signal of the control line as the procedural control. The fluorescence measurement is done using an automatic analyzer. The sensitivity and specificity given by the manufacturer in the product insert were 98% and 99% respectively. The analytical specificity did not show any sign of cross reactivity with the microorganisms evaluated (34 bacteria and 40 viruses). The specimens were also evaluated by RealStar® Adenovirus PCR Kit 1.0 (Altona diagnostics), a real time PCR that allows the detection and quantification of HAdV specific DNA. It is a CE-IVD labelled in vitro diagnostic test. The analytical sensitivity of the RealStar® Adenovirus PCR Kit 1.0 was 1.09copies/μl [95% confidence interval (CI): 0.62–3.08copies/μl]. The assay did not show cross reactivity (analytical specificity) with a panel of genomic DNA/RNA extracted from other pathogens causing similar symptoms to adenovirus infections and by testing human genomic DNA. Sixty five patients were male. The average age of the patients was 2.10 years with a range of 0.42–14 years.
A specimen was considered to be a true positive when it was confirmed by the molecular test. Medical records were reviewed in order to collect clinical and analytical data.
Statistical analysisContinuous variables were summarized as means±standard deviations (SD) or medians (interquartile range). Categorical variables were expressed as frequency and percentage. Differences between groups were assessed using Pearson's Chi square test or Fisher's exact test for categorical variables. Continuous variables were compared using independent t-test for normal data and Mann–Whitney U test for non-parametric data. All analyses were performed using SPSS software, version 19.0 (IBM Corporation, NY, U.S.) and R (https://cran.r-project.org/). All tests were calculated in a two-tailed manner and a P value of <.05 was considered statistically significant.
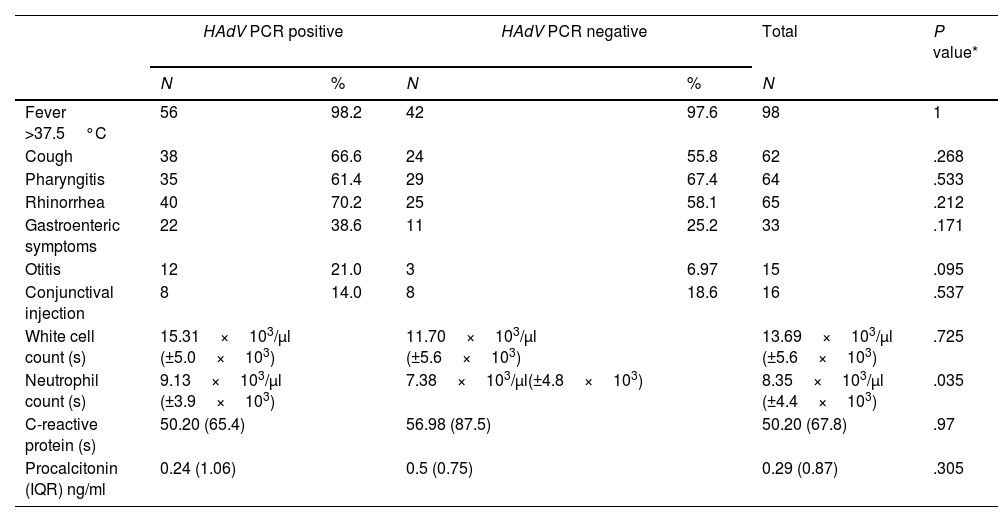
ResultsA total of 100 samples from different patients were studied. Patients were diagnosed with pharyngitis (n=64; 64.0%), rhinorrhea (n=65; 65.0%), cough (n=62; 62.0%), gastroenteritis (n=33; 33.0%), conjunctivitis (n=16; 16.0%), otitis (n=15; 15.0%), bronchiolitis (n=5; 5.0%), or pneumonia (n=5; 5.0%). Twenty six patients (26.0%) had more than one disease (pharyngitis, conjunctivitis, otitis media, bronchiolitis, pneumonia or gastroenteritis). Analysis revealed that neutrophil counts were found to be highest in patients with HAdV PCR positive (P<.05). Table 1 summarizes the clinical manifestations and analytical findings.
Clinical manifestations and analytical findings in children.
| HAdV PCR positive | HAdV PCR negative | Total | P value* | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | ||
| Fever >37.5°C | 56 | 98.2 | 42 | 97.6 | 98 | 1 |
| Cough | 38 | 66.6 | 24 | 55.8 | 62 | .268 |
| Pharyngitis | 35 | 61.4 | 29 | 67.4 | 64 | .533 |
| Rhinorrhea | 40 | 70.2 | 25 | 58.1 | 65 | .212 |
| Gastroenteric symptoms | 22 | 38.6 | 11 | 25.2 | 33 | .171 |
| Otitis | 12 | 21.0 | 3 | 6.97 | 15 | .095 |
| Conjunctival injection | 8 | 14.0 | 8 | 18.6 | 16 | .537 |
| White cell count (s) | 15.31×103/μl (±5.0×103) | 11.70×103/μl (±5.6×103) | 13.69×103/μl (±5.6×103) | .725 | ||
| Neutrophil count (s) | 9.13×103/μl (±3.9×103) | 7.38×103/μl(±4.8×103) | 8.35×103/μl (±4.4×103) | .035 | ||
| C-reactive protein (s) | 50.20 (65.4) | 56.98 (87.5) | 50.20 (67.8) | .97 | ||
| Procalcitonin (IQR) ng/ml | 0.24 (1.06) | 0.5 (0.75) | 0.29 (0.87) | .305 | ||
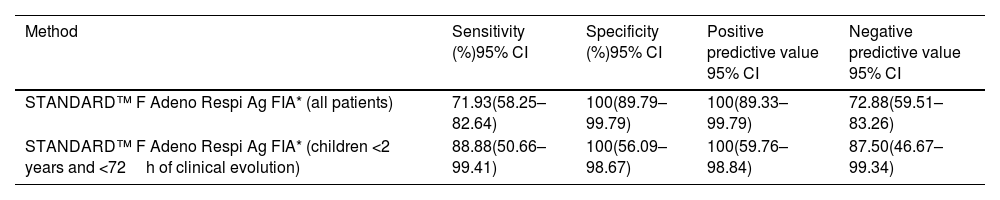
The STANDARD™ F Adeno Respi Ag FIA (IC) sensitivity was 71.93% and the specificity was 100%, using RealStar® Adenovirus PCR as the reference standard. The test performance was higher in samples from children younger than 24 months or taken less than 72h since the beginning of symptoms. In this subgroup the test showed 88.8% sensitivity and 100% specificity (Table 2). The global kappa index for the 100 samples was 0.69 (0.55–0.83). The Kappa index for the population <2 years and <72 of beginning of symptoms was 0.83 (IC 0.65–1.02).
Results of STANDARD™ F Adeno Respi Ag FIA referred to RealStar® Adenovirus PCR.
| Method | Sensitivity (%)95% CI | Specificity (%)95% CI | Positive predictive value 95% CI | Negative predictive value 95% CI |
|---|---|---|---|---|
| STANDARD™ F Adeno Respi Ag FIA* (all patients) | 71.93(58.25–82.64) | 100(89.79–99.79) | 100(89.33–99.79) | 72.88(59.51–83.26) |
| STANDARD™ F Adeno Respi Ag FIA* (children <2 years and <72h of clinical evolution) | 88.88(50.66–99.41) | 100(56.09–98.67) | 100(59.76–98.84) | 87.50(46.67–99.34) |
Differentiation of bacterial infections from viral infections is a common clinical problem. Children infected with adenovirus (HAdV) are often characterized by high-grade prolonged fever and high levels of acute-phase reactants.7 These findings are also consistent with those of bacterial infections, so HAdV infection is difficult to diagnose just by clinical symptoms. In our group of patients with HAdV infections, fever >37.5°C, rhinorrhea, cough and acute pharyngitis were the more frequent clinical manifestations but there were no statistically significative differences compared to those who had negative PCR (Table 1). These findings match in part with those of Putto et al. They analyzed 110 patients with exudative tonsillitis produced in by HAdV in 19% of the cases and group A Streptococcus in 12%, and reported that coughing and rhinitis were more likely to occur in patients with pharyngitis.7 However, in another study Takahashi et al. suggest that cough is not a good discriminator of positive and negative HAdV.8 In our study, exudative tonsillitis was not collected.
In this study, STANDARD™ F Adeno Respi Ag FIA showed sensitivity and specificity of 71.93% and 100% respectively, using RealStar® Adenovirus PCR as reference method. This low sensitivity may be attributable to the difficulty of applying an adenovirus immunochromatography assay to upper respiratory tract infections. Previous studies of this assay, performed mostly on respiratory specimens, have shown comparable specificity (91.0–100%), and variable sensitivity (54.7–95.0%).9–11 A third method to resolve discrepancies was not performed, and this can be a limitation of this study.
Some authors have reported that the positive rate of the IC test (80.4–90.0%) for specimens obtained within 4 days of the onset of illness is significantly higher than that for specimens obtained 5–11 days after onset of the illness (61.5%).8,9 In this study, 75.43% of the positive samples were examined within 96h of the onset of illness. We observed that the test sensitivity was higher if it was performed in samples from children younger than 24 months and taken less than 72h since the beginning of symptoms. In this subgroup, the test showed 88.8% sensitivity and 100% specificity, being to our criteria the optimal population for using these rapid tests in the emergency room. The NPV in the global patients is very low to make reliable therapeutic decisions (72.8%), although this NPV is higher in children <2 years and with evolution lower than 72h (87.50%). Furthermore, its use would help reduce unnecessary antibiotic use to treat possible streptococcal or other bacterial infections.4,8,12
In conclusion, STANDARD™ F Adeno Respi Ag FIA may help in a better management of pharyngitis cases in children younger than 24 months and less than 72h since the beginning of symptoms in paediatric emergency rooms.
Authors’ contributionsMPRG designed the evaluation and wrote the manuscript. BGA and PDG developed the technical work. BGA recovered the data. IBS, JGR, and ECB reviewed the manuscript.
FundingThere are not funding.
Conflicts of interestThis is a fully independent study that has not received any financial support.