Although linezolid is known to be effective when used as an adjunct therapy in the treatment of patients with multidrug-resistant tuberculosis (MDR-TB), the clinical experience is limited. In this study the efficacy and adverse effects of linezolid treatment were evaluated.
MethodsA retrospective study of tolerability and efficacy of linezolid in MDR-TB patients was performed in Madrid, Spain. Demographic characteristics, microbiological and clinical features and data on treatment tolerability were collected. Regimens were constructed with a target of prescribing, at least, five anti-tuberculosis agents likely to be effective. Linezolid, at a dosage of 1200 or 600mg daily, was included to complete the treatment if no other sensitive drugs were available. Vitamin B6 was used to reduce toxicity. Treatment outcome and clinical status at last contact were compared between patients with linezolid-containing regimens and with those without linezolid-containing regimens.
ResultsDuring the period 1998–2014, 55 patients with MDR-TB received treatment. In 21 of these patients, linezolid was added. The median of linezolid administration was 23.9 months (IQT 13.1–24.7). Patients using linezolid showed a greater resistance to drugs, with a median of 6 (IQR 5–7) compared with those who did not use it, with a median of 4 drugs (IQR 3–5) (p<0.001). The median time to sputum culture conversion of the patients in the linezolid group (73.5 days) did not differ significantly from those in the non-linezolid group (61 days) (p=0.29). There were no significant differences in the outcomes of the two patient groups. There were no reported adverse events in 81% of patients assigned to linezolid therapy. Only four patients developed toxicity attributed to linezolid. The most serious adverse event in these patients was anemia observed in the two patients treated with 1200mg per day. One of them also developed moderate paresthesia. In both cases the dosage was reduced to 600mg per day, with improvement of the anemia and paresthesias. No patients stopped linezolid therapy.
ConclusionA daily dosage of 600mg of linezolid was well tolerated without stopping treatment in any case. The efficacy of the treatment and the outcomes were similar in both the linezolid and non-linezolid group.
Aunque es conocida la utilidad y la eficacia de linezolid cuando se administra para el tratamiento de pacientes con tuberculosis multirresistente (TB-MR), la experiencia clínica actual sigue siendo limitada. En este estudio se analiza la evolución, la eficacia y los efectos adversos del tratamiento con linezolid en pacientes con diagnostico de TB-MR.
MétodoSe realizó un estudio retrospectivo sobre la tolerabilidad y la eficacia de linezolid en pacientes diagnosticados de TB-MR en Madrid, España. Los regimenes de tratamiento se constituyeron con al menos 5 fármacos antituberculosos efectivos. Linezolid (a dosis de 1200 o 600mg por día) se añadió para completar el tratamiento en los pacientes en los que no existían otros fármacos alternativos sensibles. Se utilizó vitamina B6 para reducir la toxicidad al tratamiento. Se comparó la evolución clínica de los pacientes y los resultados del tratamiento entre el grupo de pacientes tratados con linezolid y el grupo de pacientes que no fueron tratados con este fármaco.
ResultadosEntre 1998 y 2014, 55 pacientes fueron diagnosticados de TB-MR y recibieron tratamiento. En 21 pacientes fue necesaria la administración de linezolid. La media de tiempo de administración de linezolid fue de 23.9 meses (IQR 13.1-24.7). Los pacientes del grupo de linezolid presentaron mayor número de resistencias a fármacos con una mediana de 6 (IQR 5-7), respecto al grupo que no tomó Linezolid [4 fármacos (IQR 3-5)] (p<0,001). No hubo diferencias significativas entre el tiempo de conversión del cultivo de esputo de los pacientes del grupo de linezolid (73,5 días) frente al del grupo sin linezolid (61 días) (p=0,29). Tampoco hubo diferencias significativas en la evolución de los dos grupos de pacientes. El 81% de los pacientes que tomaron linezolid no experimentaron efectos adversos atribuidos a la administración de Linezolid. Sólo cuatro pacientes desarrollaron toxicidad secundaria al uso de linezolid. El efecto adverso más importante fue anemia que se presentó en los dos pacientes tratados con 1200mg al día. Uno de ellos también desarrolló parestesias de grado moderado en miembros inferiores. En ambos casos, la dosis se redujo a 600mg al día, con mejoría de la anemia y de las parestesias.
ConclusiónLa dosis de 600mg al día de linezolid fue bien tolerada sin necesidad de interrumpir el tratamiento en ningún caso. La eficacia del tratamiento y la evolución de los pacientes fueron similares en ambos grupos.
Linezolid is the first oxazolidinone approved for clinical use, which has proved to be effective when added to the treatment of patients with multidrug-resistant tuberculosis (MDR-TB). Linezolid inhibits protein synthesis by binding to the 50S ribosomal subunit of mycobacteria. The drug is rapidly absorbed and well distributed in tissues. The minimal inhibitory concentration of linezolid to Mycobacterium tuberculosis is 0.5–1μg/ml.1 Although linezolid has a moderate early bactericidal activity against M. tuberculosis, it has shown to have excellent efficacy in vitro as well as in mouse model studies against M. tuberculosis, including multidrug-resistant and extensively resistant strains.1 In spite of this discrepancy, linezolid has been used successfully in the treatment of patients with MDR-TB and XDR-TB, with favorable outcome in up to 73–80% of the patients. However, the information was mainly based on retrospective data on patient material.2,3 Nevertheless these good results, its high price in many countries and the appearance of adverse effects, such as bone marrow toxicity or neuropathy, have resulted in the avoidance of its widespread use. Due to these side events, treatment discontinuation has been necessary in many cases, reaching up to 77% of the patients. When administered in a smaller dosage, an important decrease of adverse effects has been observed and no differences have been found in the patient's outcome.2
The aim of this study was to analyze the tolerability and efficacy of the administration of linezolid, when it is used as part of a multidrug-resistant tuberculosis treatment and to compare it with a no linezolid-containing regimen.
Materials and methodStudy patientsA retrospective study of a series of multidrug-resistant (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) diagnosed patients was performed in Madrid. We included patients older than 17 years. Standard definitions were used for MDR-TB (i.e., tuberculosis with confirmed phenotypic resistance to at least isoniazid and rifampicin) and XDR-TB-MR (i.e., MDR-TB with additional resistance to any fluoroquinole and at least, 1 of the 3 injectable drugs: capreomycin, kanamycin or amikacin). The patients came from other hospitals, emergency department or primary care in Madrid and they were admitted in the Isolation Ward of Internal Medicine of La Paz-Cantoblanco Hospital in Madrid. At first, the physician responsible performed a clinical questionnaire. Data included demographic characteristics, comorbidity conditions, clinical features and radiographic information.
Microbiological dataSputum staining and culture were done monthly during the first 6 months and once every two months afterwards until treatment completion. Sputum culture conversion was defined as two consecutive negative sputum samples on solid (Lowenstein–Jensen) medium in patients who were sputum culture positive at diagnosis. Time to culture conversion was defined as time from treatment start to date of the first of two consecutive negative cultures. Surgery was performed in cases of localized disease refractory to medical treatment.
Sputum smear, culture and drugs-susceptibility testing (DST) for first- and second-line drugs of all patients were tested by absolute concentration method on Lowenstein–Jensen medium, in three different microbiology departments (National Center of Microbiology of Majadahonda, in the Mycobacterial Microbiology Department of Gregorio Marañón and La Paz Hospital in Madrid). The MIC of linezolid against M. tuberculosis was 1μg/ml.
Treatment regimensRegimens were constructed in an individualized treatment determined on the basis of DST results and the prior treatment history. Treatments consisted of four to six active first- or second-line anti-tuberculosis drugs (pyrazinamide and ethambutol whenever it was possible, one injectable agent, one fluoroquinolone and two or more second-/third-line anti-tuberculosis drug).
Linezolid was included when additional drug was needed to complete the treatment and no other sensitive drugs were available. It was administered orally at a dosage of 600mg to all, but two patients received doses of 600mg twice a day.
Vitamin B6 was administered simultaneously to all the patients taking linezolid, in order to prevent hematologic toxicity and peripheral neuropathy.
As a standard, treatment was administered between 18 and 24 months, in a directly observed manner. The compliance to treatment was ensured during the hospitalization, and after discharge, a team of Red Cross Organization in Madrid verified daily the drug administration.
Adverse-event monitoringMyelosuppression, peripheral neuropathy and optic neuropathy were assessed in this analysis. A toxicity questionnaire was completed daily during inpatient study and then every two months when they were outpatients. Complete blood count and routine blood chemistry were performed every 2 weeks during the hospitalization and then every two months until treatment completion.
Mild adverse effect was defined when their presence did not involve any change of drug administration, Moderate when it was necessary to decrease the dose of the drug or dispense ancillary drugs and Severe when the replacement for another anti-tuberculosis drug was found necessary. We assessed anemia as mild (hemoglobin level, 10–13g/dL), moderate (hemoglobin level, 8–9g/dL), or severe (<8g/dL).
Outcome measuresWe examined the treatment outcome by clinical and radiographic improvement and by sputum smear and culture conversion. After discharge, the patients were screened as outpatient once every two months with the same doctor throughout the course of treatment. A chest radiograph was performed every 6 months. The International Definitions of treatment Outcomes, based on the recommendations of the Word Health Organization, were used to define the treatment outcome of both groups and included cured, treatment completion, still on treatment, failure, died, and defaulted.4 Patients who completed treatment were being followed for recurrent disease for the next two years. Treatment outcome and clinical status at last contact were compared between patients with linezolid-containing regimens and with those without linezolid-containing regimens.
Because this study used routinely collected surveillance and treatment data without patient identifiers institutional review board approval was not required.
Statistic analysisWe analyzed the baseline characteristics, resistance pattern, sputum smear conversion, treatment outcome and clinical status at last contact of multidrug-resistant tuberculosis patients with linezolid-containing regimens compared with those with no linezolid-containing regimens. Categorical variables were expressed as numbers (percentage) and the continuous variables as median (interquartile range). Categorical variables were compared using the Chi-square test and continuous variables were compared using the Mann–Whitney U test. A p<0.05 was considered significant. SPSS 11.0 software was used for analysis.
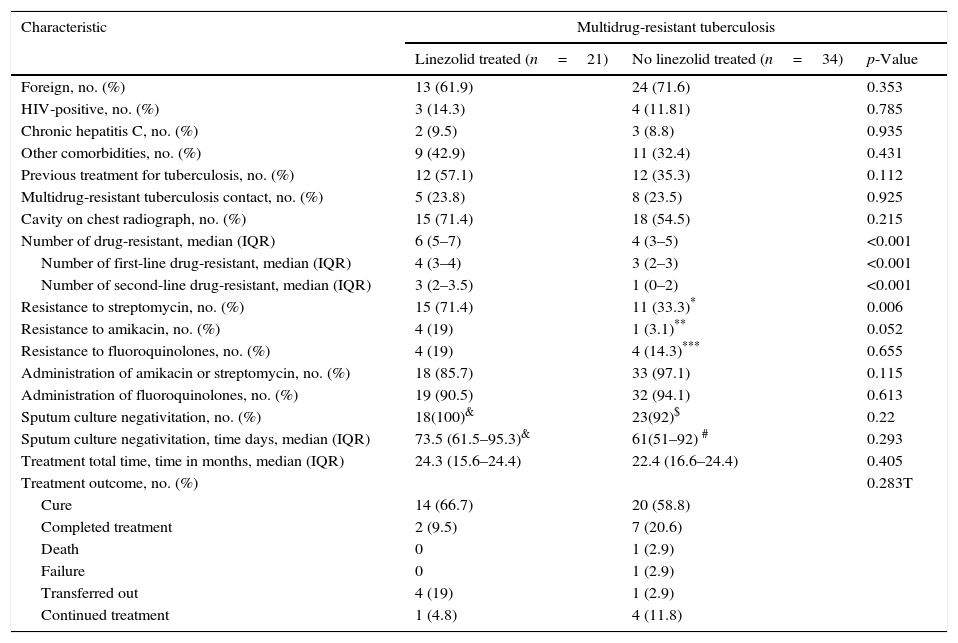
ResultsDemographic characteristicsFrom January 1998 to February 2014, 55 patients had a MDR-TB diagnosis. Twenty-one patients were treated with linezolid as part of the multidrug regimen for MDR-TB. No differences in demographic features were found between patients who were using linezolid and those who were not using it. In the linezolid group, the majority were males 14 (66.7%) and the mean age was 37 years old (DS 14.4). 15 patients (71.4%) had cavitary on chest radiograph.
57% of patients treated with linezolid had a history of previous treatment for tuberculosis vs 35.3% of patients in the no linezolid group (p=0.112). Patients in the linezolid group presented a greater resistance to drugs with a median of 6 (IQR 5–7) vs 4 (IQR 3–5) compared with the other group (p<0.001). Characteristics of the patients are shown in Table 1.
Characteristics, resistance pattern and sputum smear conversion of multidrug-resistant tuberculosis.
| Characteristic | Multidrug-resistant tuberculosis | ||
|---|---|---|---|
| Linezolid treated (n=21) | No linezolid treated (n=34) | p-Value | |
| Foreign, no. (%) | 13 (61.9) | 24 (71.6) | 0.353 |
| HIV-positive, no. (%) | 3 (14.3) | 4 (11.81) | 0.785 |
| Chronic hepatitis C, no. (%) | 2 (9.5) | 3 (8.8) | 0.935 |
| Other comorbidities, no. (%) | 9 (42.9) | 11 (32.4) | 0.431 |
| Previous treatment for tuberculosis, no. (%) | 12 (57.1) | 12 (35.3) | 0.112 |
| Multidrug-resistant tuberculosis contact, no. (%) | 5 (23.8) | 8 (23.5) | 0.925 |
| Cavity on chest radiograph, no. (%) | 15 (71.4) | 18 (54.5) | 0.215 |
| Number of drug-resistant, median (IQR) | 6 (5–7) | 4 (3–5) | <0.001 |
| Number of first-line drug-resistant, median (IQR) | 4 (3–4) | 3 (2–3) | <0.001 |
| Number of second-line drug-resistant, median (IQR) | 3 (2–3.5) | 1 (0–2) | <0.001 |
| Resistance to streptomycin, no. (%) | 15 (71.4) | 11 (33.3)* | 0.006 |
| Resistance to amikacin, no. (%) | 4 (19) | 1 (3.1)** | 0.052 |
| Resistance to fluoroquinolones, no. (%) | 4 (19) | 4 (14.3)*** | 0.655 |
| Administration of amikacin or streptomycin, no. (%) | 18 (85.7) | 33 (97.1) | 0.115 |
| Administration of fluoroquinolones, no. (%) | 19 (90.5) | 32 (94.1) | 0.613 |
| Sputum culture negativitation, no. (%) | 18(100)& | 23(92)$ | 0.22 |
| Sputum culture negativitation, time days, median (IQR) | 73.5 (61.5–95.3)& | 61(51–92) # | 0.293 |
| Treatment total time, time in months, median (IQR) | 24.3 (15.6–24.4) | 22.4 (16.6–24.4) | 0.405 |
| Treatment outcome, no. (%) | 0.283T | ||
| Cure | 14 (66.7) | 20 (58.8) | |
| Completed treatment | 2 (9.5) | 7 (20.6) | |
| Death | 0 | 1 (2.9) | |
| Failure | 0 | 1 (2.9) | |
| Transferred out | 4 (19) | 1 (2.9) | |
| Continued treatment | 1 (4.8) | 4 (11.8) | |
T favorable treatment outcome (cure and completed treatment) vs unfavorable treatment outcome (death and failure). IQR; interquartile range. Significant result (p<0.05).
Time calculated on 23 patients (seven patients had negative sputum culture at the start of effective treatment, two patients with extrapulmonary tuberculosis and two patients without negative sputum culture).
Each regimen included a fluorquinolone and an injectable agent unless resistance was noted to these classes of drugs. The median duration of linezolid administration was 23.9 months (IQT 13.1–24.7). Two patients had a XDR-TB diagnosis; both had a positive culture to Mycobacterium bovis with resistance to 9 drugs. One received linezolid and another one did not take the drug. One patient from the linezolid group underwent segmental pneumonectomy. This patient achieved negative culture after two months and was discharged to another hospital
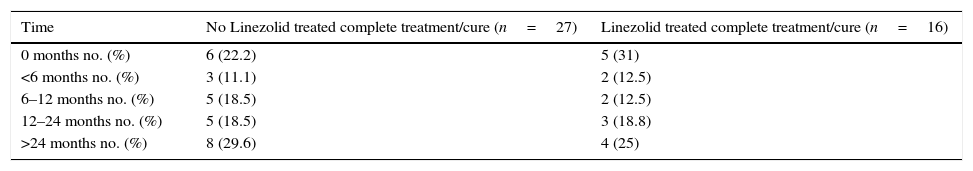
EfficacyThere was no difference in terms of length of hospital stay, 2.98 months (IQR 1.88–4.72) for the linezolid group vs 2.83 months (IQR 2.07–4.73) for the non-linezolid group (p=0.86). Time to sputum culture conversion was slightly longer in patients treated with linezolid (73.5 days, IQR 61.5–95.6) than in no linezolid group (61 days, IQR 51–92), however, this difference was no significant (p=0.29). Likewise, there were no significant differences in the outcome of the two patient groups (Table 1). At data censure (1 February 2014), 16 out of 21 patients, from the linezolid group, achieved the cure criteria or completed the treatment. Four of them were transferred and 1 was still receiving treatment with a favorable microbiological and clinical outcome. Patients, who completed treatment, continued monitoring outside and remained asymptomatic (Table 2).
Outcomes of patients who completed treatment and remained asymptomatic.
| Time | No Linezolid treated complete treatment/cure (n=27) | Linezolid treated complete treatment/cure (n=16) |
|---|---|---|
| 0 months no. (%) | 6 (22.2) | 5 (31) |
| <6 months no. (%) | 3 (11.1) | 2 (12.5) |
| 6–12 months no. (%) | 5 (18.5) | 2 (12.5) |
| 12–24 months no. (%) | 5 (18.5) | 3 (18.8) |
| >24 months no. (%) | 8 (29.6) | 4 (25) |
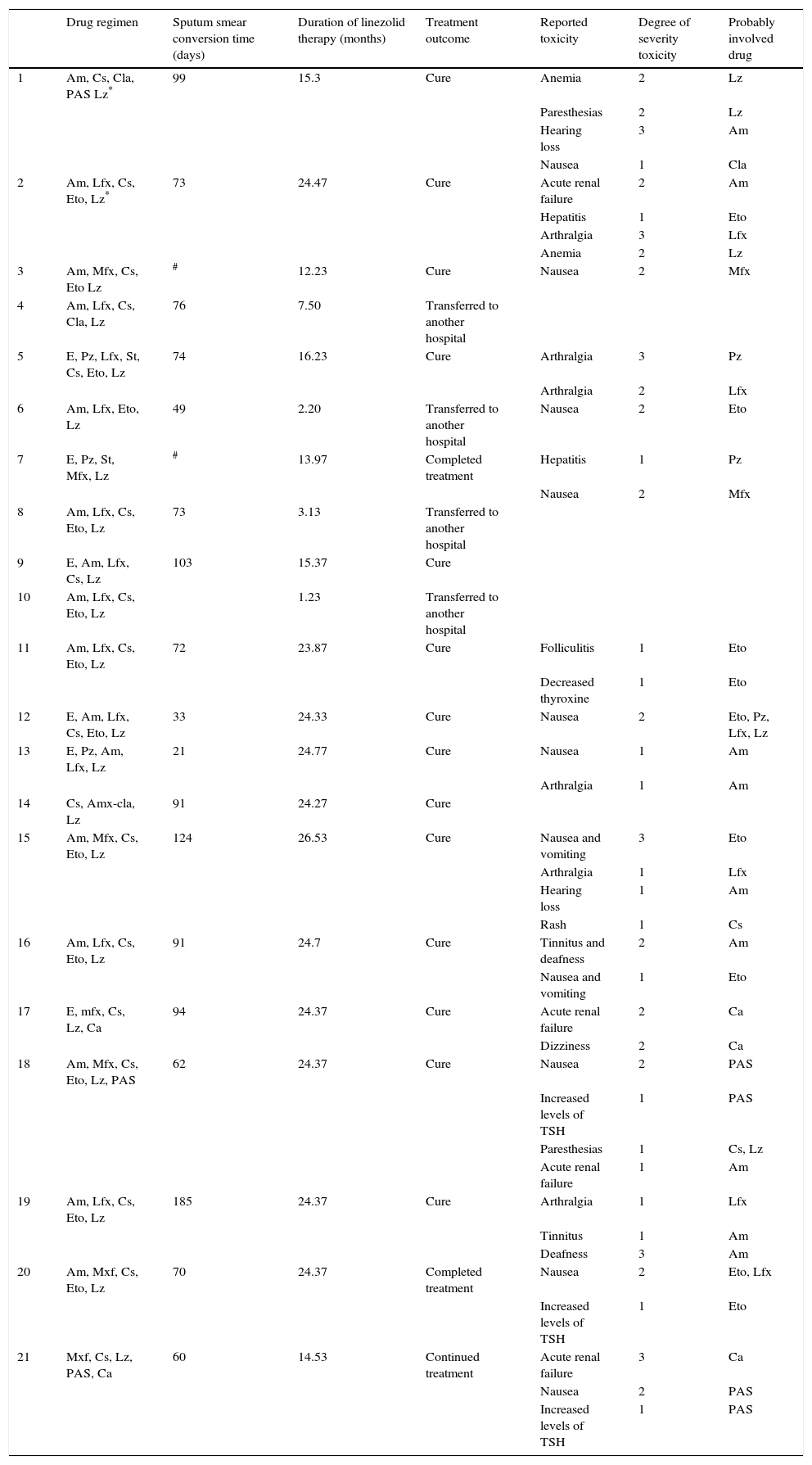
Of the 21 linezolid treated patients, 17 (81%) had no reported toxicity attributed to linezolid therapy. Two patients developed anemia with a dosage of 1200mg per day and one of them had paresthesias. The dosage was reduced to 600mg per day. As a result there was an improvement of the anemia and paresthesias. One patient had paresthesias with a dosage of 600mg per day and another had nausea, but this patient was treated with another four drugs that frequently produced nausea as adverse effects (ethionamide, levofloxacin and pyrazinamide). No patients stopped linezolid therapy because of adverse effects. The remaining patients tolerated well daily doses of 600mg and there was no evidence of other side effects linked to this drug during its administration period. The toxicities and the treatment outcome from the patients with multidrug-resistant tuberculosis with linezolid-containing regimens are shown in Table 3.
Toxicities and treatment outcome of cases of multidrug-resistant tuberculosis patients with linezolid-containing regimens.
| Drug regimen | Sputum smear conversion time (days) | Duration of linezolid therapy (months) | Treatment outcome | Reported toxicity | Degree of severity toxicity | Probably involved drug | |
|---|---|---|---|---|---|---|---|
| 1 | Am, Cs, Cla, PAS Lz* | 99 | 15.3 | Cure | Anemia | 2 | Lz |
| Paresthesias | 2 | Lz | |||||
| Hearing loss | 3 | Am | |||||
| Nausea | 1 | Cla | |||||
| 2 | Am, Lfx, Cs, Eto, Lz* | 73 | 24.47 | Cure | Acute renal failure | 2 | Am |
| Hepatitis | 1 | Eto | |||||
| Arthralgia | 3 | Lfx | |||||
| Anemia | 2 | Lz | |||||
| 3 | Am, Mfx, Cs, Eto Lz | # | 12.23 | Cure | Nausea | 2 | Mfx |
| 4 | Am, Lfx, Cs, Cla, Lz | 76 | 7.50 | Transferred to another hospital | |||
| 5 | E, Pz, Lfx, St, Cs, Eto, Lz | 74 | 16.23 | Cure | Arthralgia | 3 | Pz |
| Arthralgia | 2 | Lfx | |||||
| 6 | Am, Lfx, Eto, Lz | 49 | 2.20 | Transferred to another hospital | Nausea | 2 | Eto |
| 7 | E, Pz, St, Mfx, Lz | # | 13.97 | Completed treatment | Hepatitis | 1 | Pz |
| Nausea | 2 | Mfx | |||||
| 8 | Am, Lfx, Cs, Eto, Lz | 73 | 3.13 | Transferred to another hospital | |||
| 9 | E, Am, Lfx, Cs, Lz | 103 | 15.37 | Cure | |||
| 10 | Am, Lfx, Cs, Eto, Lz | 1.23 | Transferred to another hospital | ||||
| 11 | Am, Lfx, Cs, Eto, Lz | 72 | 23.87 | Cure | Folliculitis | 1 | Eto |
| Decreased thyroxine | 1 | Eto | |||||
| 12 | E, Am, Lfx, Cs, Eto, Lz | 33 | 24.33 | Cure | Nausea | 2 | Eto, Pz, Lfx, Lz |
| 13 | E, Pz, Am, Lfx, Lz | 21 | 24.77 | Cure | Nausea | 1 | Am |
| Arthralgia | 1 | Am | |||||
| 14 | Cs, Amx-cla, Lz | 91 | 24.27 | Cure | |||
| 15 | Am, Mfx, Cs, Eto, Lz | 124 | 26.53 | Cure | Nausea and vomiting | 3 | Eto |
| Arthralgia | 1 | Lfx | |||||
| Hearing loss | 1 | Am | |||||
| Rash | 1 | Cs | |||||
| 16 | Am, Lfx, Cs, Eto, Lz | 91 | 24.7 | Cure | Tinnitus and deafness | 2 | Am |
| Nausea and vomiting | 1 | Eto | |||||
| 17 | E, mfx, Cs, Lz, Ca | 94 | 24.37 | Cure | Acute renal failure | 2 | Ca |
| Dizziness | 2 | Ca | |||||
| 18 | Am, Mfx, Cs, Eto, Lz, PAS | 62 | 24.37 | Cure | Nausea | 2 | PAS |
| Increased levels of TSH | 1 | PAS | |||||
| Paresthesias | 1 | Cs, Lz | |||||
| Acute renal failure | 1 | Am | |||||
| 19 | Am, Lfx, Cs, Eto, Lz | 185 | 24.37 | Cure | Arthralgia | 1 | Lfx |
| Tinnitus | 1 | Am | |||||
| Deafness | 3 | Am | |||||
| 20 | Am, Mxf, Cs, Eto, Lz | 70 | 24.37 | Completed treatment | Nausea | 2 | Eto, Lfx |
| Increased levels of TSH | 1 | Eto | |||||
| 21 | Mxf, Cs, Lz, PAS, Ca | 60 | 14.53 | Continued treatment | Acute renal failure | 3 | Ca |
| Nausea | 2 | PAS | |||||
| Increased levels of TSH | 1 | PAS |
Drug regimen: Pz, pyrazinamide; E, ethambutol; Lfx, levofloxacin; Mfx, moxifloxacin; Am, amikacin; St, streptomycin; Cs, cycloserine; Eto, ethionamide; Lz, linezolid; Amx-cla, amoxicillin-clavulanate; Cla, clarithromycin; Capreomycin, Ca.
Degree of severity toxicity: 1, mild; 2, moderate; 3, severe.
Although the group with Linezolid therapy had a significantly greater resistance to drugs, there were no differences in their outcome, nor in the time of sputum culture conversion or the time of hospital stay compared with no linezolid group. Time of sputum culture conversion was 73.5 days, shorter when compared to other retrospective reviews.2,5 The high percentage of patients taking fluoroquinolones and injectable agents probably had an influence on this result.5
Among the present patients, the administration of linezolid was associated with a low incidence of adverse effects. Long-term linezolid administration has reported major adverse effects, like myelosuppression and peripheral and optic neuropathy. These are limiting factors in the use of linezolid, because it implies the discontinuation of the drug administration in a large proportion of patients.2,3,6–8 Cox et al. in a meta-analysis, reported that the pooled proportions of the frequency of neuropathy and bone marrow toxicity were 36% and 28% respectively,9 and the proportion of patients in whom linezolid was stopped ranged from 6% to 79%.6–8 Soutgiu et al. observed in another meta-analysis side events in 59% of the patients, and 68% of these were notified as major.10 Moreover, other adverse effects related to linezolid have been described, such as lactic acidosis, gastrointestinal intolerance and serotonin syndrome.11
Despite the fact that in our series there were simultaneous comorbidities, and patients received linezolid for a long time (23.9 months of median), only four patients, two of them receiving a 1200mg daily dosage, showed adverse effects. Discontinuance of the drug administration was not necessary in these cases and two patients recovered when the dose was reduced to 600mg per day. Our data show similarities to Singla et al. study, in which, only five patients, who were treated with linezolid (17%), had severe side effects. From these patients only three, who were receiving high dose linezolid (600mg twice a day), required discontinuation of the therapy. Minor adverse effects were managed by temporal discontinuation or by the use of ancillary drugs.12
Other research studies have shown how patients who were administered a smaller dosage of linezolid developed minor side effects. A review of eight studies showed a lower frequency of adverse events in patients who were administered a 600mg daily dosage (34% vs 50% toxicity shown by patients who were administered 1200mg per day).6 Yet, the success of the 600mg per day regimen (67%) did not differ from the regimens using 1200mg per day (62%). In a Korean study of XDR-TB, linezolid at doses of 600mg or 300mg daily was added to the XDR-TB regimen. 89% of the linezolid group patients achieved sputum conversion after 6 months, with a mean of 75 days after initiation of treatment with linezolid. Only 11% of the patients developed acquired resistance to linezolid, even though linezolid was added to a failing regimen.13 This low frequency of resistance acquisition may be related to the low rate of resistance mutations observed in vitro. A daily dose of 600mg might seem to maintain concentrations that would prevent the emergence of resistance.
The low dose of linezolid used in the present subjects, together with vitamin B6 administration as well as the close monitoring of the adverse effects, were probably reasons for the low degree of toxicity observed.14
Our study is associated with limitations including the low number of patients and the retrospective nature. A limitation of retrospective studies is the difficulty to control the effect of other drugs. And another inherent trouble on series of TB patients is the fact that patients with social problems are included in it, which implies a lack of an appropriate monitoring.
ConclusionsOur collected data suggest a 600mg daily linezolid dosage as safe, with rare adverse effects. Similarly, neither the efficacy of the treatment nor the evolution of MDR-TB patients was affected.
Further research done with a larger number of patients and randomized clinical trials are necessary in order to determine specifically the optimum dosage tolerated while remaining effective.
Conflict of interestThe authors declare no conflict of interest.