In Spain, minors represent approximately 20% of the immigration flow. Many of these immigrants come from countries in the tropics and sub-tropics where intestinal parasitic infections caused by helminths and protozoa are one of the major causes of human disease. The main objective of the present work was to describe parasite infections in a group of immigrant children.
MethodsA prospective evaluation was performed in 373 minors from Sub-Saharan Africa, North Africa, and Latin America. Details were collected from the medical records and physical examination. Urine, stool and peripheral blood samples were obtained for serological and routine laboratory tests. Direct and indirect parasitological tests were also performed.
ResultsAt least 1 parasitic disease was diagnosed in 176 (47.1%) immigrant children, while 77 (20.6%) minors were infected with two or more parasites. The number of parasites was highest in children from Sub-Saharan Africa compared with the rest of the areas of origin (p<.001), and in children from urban areas compared with those from rural areas (OR 1.27 [1.059–1.552], p=.011). The most frequent causes of multiple parasite infection were filariasis plus strongyloidiasis and filariasis plus schistosomiasis. Intestinal parasite infection was diagnosed in 38 cases (13.8%). Logistic regression analysis revealed that for each month of stay, the probability of a positive finding in the stool sample decreased by 0.02% [β=−0.020, (p=.07)].
ConclusionsThe high infection rates of parasite diseases in immigrant children point to the need for screening protocols for certain infectious diseases in these children according to their country of origin and their length of residence in Spain.
En España, los menores representan aproximadamente el 20% del flujo migratorio. Muchos de estos menores provienen de regiones tropicales y subtropicales donde las infecciones por helmintos y protozoos son una de las principales causas de morbilidad. El objetivo de este trabajo es describir las infecciones parasitarias presentes en un colectivo de menores inmigrantes.
MétodosSe evaluaron prospectivamente 373 menores procedentes de África subsahariana, África del Norte y Latinoamérica. Se realizó una historia clínica detallada. Se obtuvieron muestras de sangre periférica, orina y heces para la realización de los diferentes análisis bioquímicos, serológicos y parasitológicos directos e indirectos.
ResultadosEn 176 (47,1%) menores se diagnosticó al menos una enfermedad parasitaria. En 77 (20,6%) menores se detectaron 2 o más parásitos. En los niños de África subsahariana el número de parásitos fue mayor comparado el resto de orígenes (p<0,001). Los menores de zonas urbanas tenían más parásitos comparado con los niños de zonas rurales (OR 1,27 [1059-1552], p=0,011). Las causas más frecuentes de parasitación múltiple fueron filariosis más estrongiloidosis y filariosis más esquistosomiasis. Se diagnosticó parasitosis intestinal en 38 casos (13,8%). El análisis de regresión logística reveló que por cada mes de estancia, la probabilidad de un resultado positivo en las heces disminuía un 0,02% [β=−0,020 (p=0,07)].
ConclusiónLas altas tasas de infección parasitaria en niños inmigrantes señala la necesidad de una detección protocolizada de estas enfermedades según el país de origen y el tiempo de residencia en España.
The migratory flow from low-income countries located in tropical and subtropical areas to developed countries is continuously increasing. Therefore, the health status of the immigrant population has become a relevant subject in developed countries.
Frequently, these immigrants present with imported infectious diseases. Some of the most frequent diseases are caused by parasites, usually helminths.1–3 Often, these infections are asymptomatic or have nonspecific symptoms. Although the overall mortality from these infections is low, parasite infections are among the main causes of morbidity in low-income countries. Therefore, some infections (i.e., lymphatic filariasis, schistosomiasis or onchocerciasis) have been identified by the WHO as important causes of morbidity and have a high disease burden.4–6
Therefore, healthcare professionals must be aware of issues pertaining to screening, diagnostics, and treatment for diseases that are not endemic. This can be challenging, particularly with shifting patterns of migration and resultant changes in disease epidemiology.7
In Spain, as in other European countries, almost 20% of new immigrants are minors.8 However, little data are available in the medical literature concerning imported parasitic diseases in immigrant minors in Europe.9,10 In international adoptees infectious conditions of special concern include presence of intestinal parasites.11 Thus, appropriate medical screening in this high-risk population is not well established.9
The main objective of the present study was to describe the imported parasitic diseases in a collective of immigrant minors from Sub-Saharan Africa, North Africa, and Latin America.
Patients and methodsThis study was performed in the Tropical Medicine Office (TMO) of Complejo Asistencial Universitario of Salamanca (CAUSA), Salamanca, Spain. We prospectively evaluated the prevalence of parasitic diseases through screening programs in immigrants under 18 years of age coming from Sub-Saharan Africa, North Africa and Latin America between January 2007 and December 2011. The study was reviewed and approved by the ethical committee of CAUSA, and written consent was obtained from the subjects’ legal guardians. Minors or guardians were asked about where they came from to define urban or rural area. In case of doubt the cutoff of 5000 as rural inhabitants and more than 5000 inhabitants as urban was applied. Two categories in terms of the immigrants’ lengths of residence were considered: recently arrived immigrants, defined as people with less than six months in Spain, and immigrants of long stay, defined as people with ≥6 months in our country, including those who travel occasionally to their origin countries.
The immigrant minors were screened by the examination of detailed medical records and a physical examination. Urine and peripheral blood samples were obtained from patients for serological and routine laboratory tests. Anemia was defined as a hemoglobin level ≤11.5g/dL. Eosinophilia was defined as >0.45×109eosinophils/L of blood. Direct parasitological tests included: (i) examination of 3 stool specimens taken 48–72h apart were requested from each child. Specimens were preserved in 10% formalin and polyvinyl alcohol. Samples in formalin were concentrated; (ii) microscopy of a terminal urine specimen; in selected patients; (iii) 24h urine sample for testing ova, (iv) Knott test for microfilaremia and (v) skin snips. Two skin snips were taken from each patient, one from the lateral aspect of each buttock. Each snip measured 1–2mm and to form a diamond shaped grid of about 2cm×2cm on each buttock. Indirect parasitological tests included commercial serologic tests for Echinococcus granulosus (Echinococcosis Fumouze, Fumouze Diagnostics, France), Taenia solium (NovaLisa Taenia solium IgG, NovaTec ImmunDiagnostica GmbH, Germany) and Trypanosoma cruzi (Architect Chagas, Abbott Laboratories, USA). In-house ELISA assays were used for the diagnosis of filariasis, schistosomiasis, fasciolosis, and strongyloidiasis.12–14
Statistical analysisThe descriptive data analysis was expressed as the mean plus standard deviation (SD) and percentages when appropriate. One-way ANOVA was used to compare analytical values among the three origin areas. The χ2 test was used to test associations between categorical variables and specifically to evaluate the association between demographic variables and the final diagnoses. A regression analysis was performed to investigate associations between length of stay and the probability of a positive parasitology results. The level of statistical significance was p<0.05. SPSS 21 statistical software (available from http://www.spss.com) was used for the statistical analyses.
Ethics statementThis study was approved by the Ethics Committee of Complejo Asistencial Universitario de Salamanca (CAUSA). Written informed consent was obtained from legal guardians. All data analyzed were anonymized.
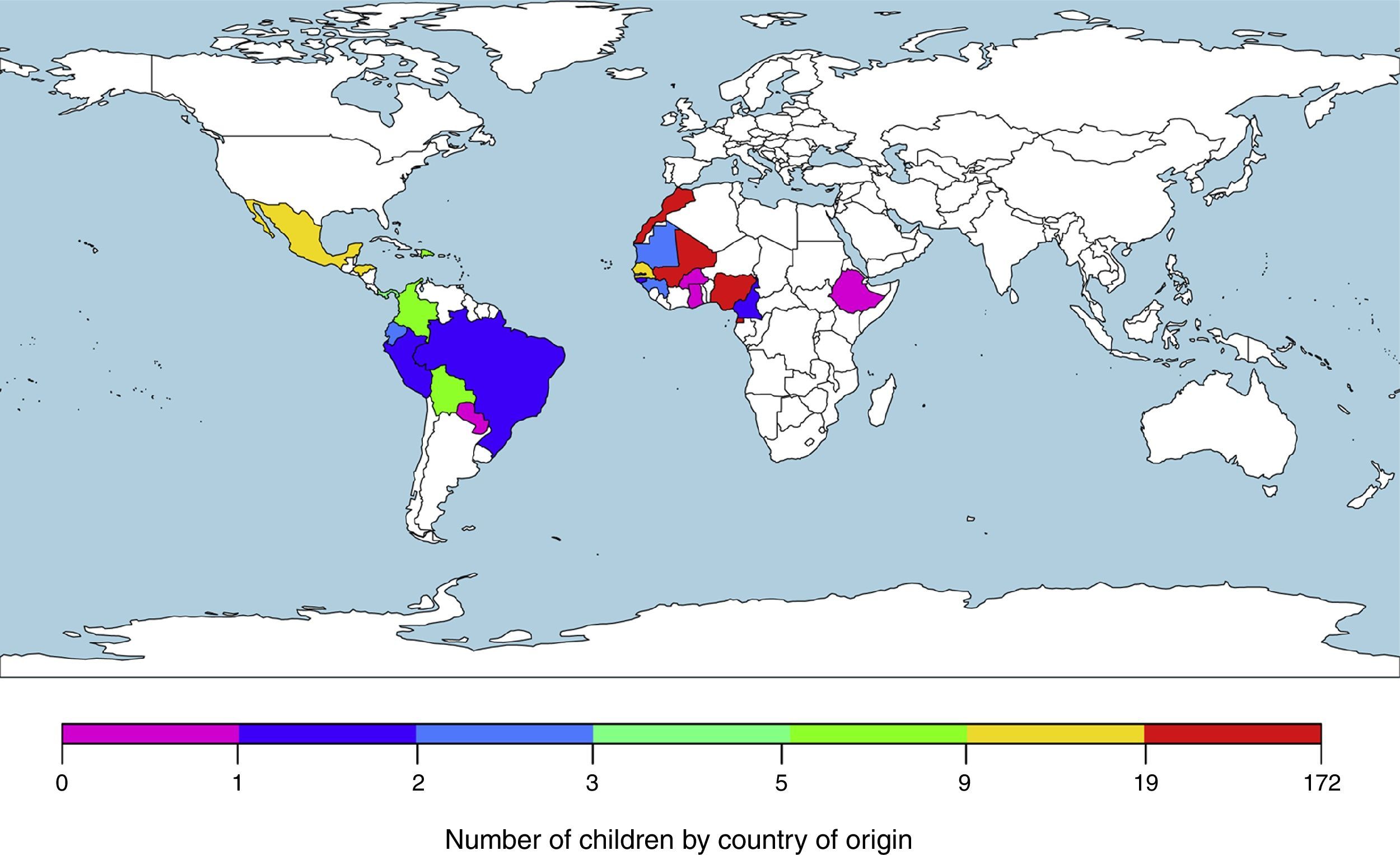
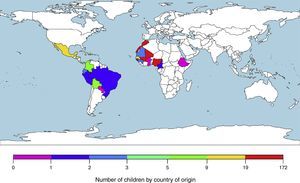
ResultsWe included 373 patients from tropical and subtropical areas. The majority of the subjects originated from Sub-Saharan Africa (250/373; 67.0%), followed by North Africa (67/373; 18.0%) and Latin America (56/373; 15.0%). Fig. 1 details the origin countries of the children included in the study. Additionally, the main demographic data of the participants categorized by origin areas are shown in Table 1.
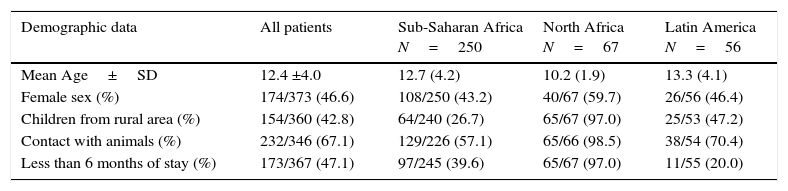
Principal demographic data of the participants included in the study.
| Demographic data | All patients | Sub-Saharan Africa N=250 | North Africa N=67 | Latin America N=56 |
|---|---|---|---|---|
| Mean Age±SD | 12.4 ±4.0 | 12.7 (4.2) | 10.2 (1.9) | 13.3 (4.1) |
| Female sex (%) | 174/373 (46.6) | 108/250 (43.2) | 40/67 (59.7) | 26/56 (46.4) |
| Children from rural area (%) | 154/360 (42.8) | 64/240 (26.7) | 65/67 (97.0) | 25/53 (47.2) |
| Contact with animals (%) | 232/346 (67.1) | 129/226 (57.1) | 65/66 (98.5) | 38/54 (70.4) |
| Less than 6 months of stay (%) | 173/367 (47.1) | 97/245 (39.6) | 65/67 (97.0) | 11/55 (20.0) |
The minors included in the study were usually asymptomatic (49%) or reported nonspecific symptoms; the most frequently observed symptoms were digestive symptoms (11%; abdominal pain and occasional diarrhea) and dermatological (6%; pruritus). The physical examination was unremarkable in 45.8% of the children, while 23.8% of cases presented with dermatological lesions, and 2% showed liver and spleen enlargement. Eighty-three children (22.3%) were diagnosed with parasitic infection-related eosinophilia (p<0.05) [eosinophils median±IQR (eosinophils/L) 184.0±320.0] and twenty-one (5.6%) were diagnosed with anemia, representing the most frequent hematologic disorders.
At least one parasitic infection was diagnosed in 176 out of 373 (47.1%) immigrant children, with the most frequent diagnoses in minors from Sub-Saharan Africa (142; 57%) compared to the Northern African (19; 27.9%) or Latin American (16; 28.5%) patients (p<0.001). Moreover, we detected two or more parasites in 77 out of 373 (20.6%) children according to our previous data, with the detection more frequent in minors from Sub-Saharan Africa [67 (26.8%) patients] compared to Northern Africa and Latin America [5 (7.3%) and 2 (3.5%) patients, respectively, p<0.001]. The number of parasites detected was higher in children from urban areas compared to rural areas (OR 1.27 [1.059–1.552], p=0.011). The most frequent causes of multiple parasites were filariasis plus strongyloidiasis and filariasis plus schistosomiasis.
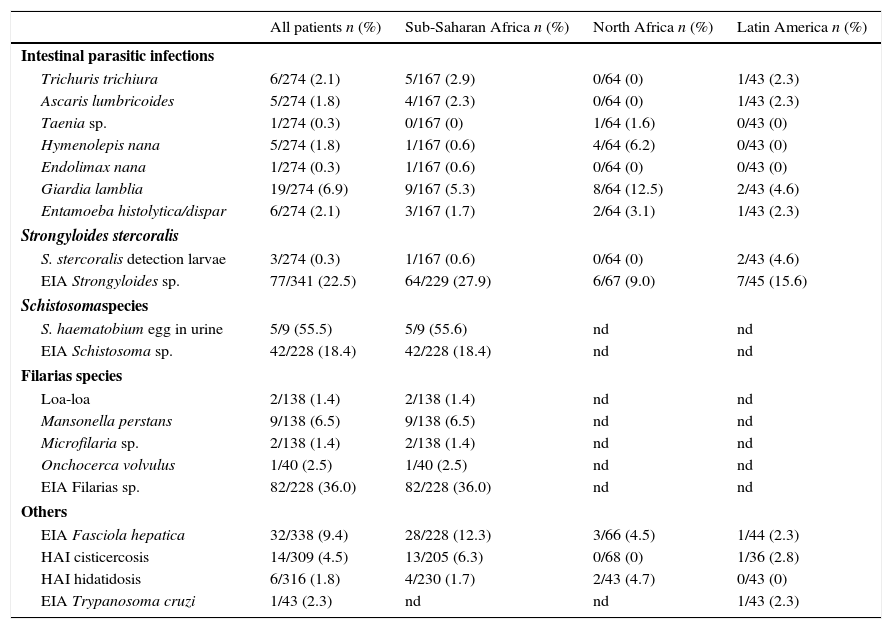
The final diagnosis and the number of tests used are shown in Table 2. Two hundred and seventy-four cases were investigated for stool ova and parasites. Intestinal parasitic infection was diagnosed in 38 (13.8%) patients. The main infections detected after examination of stool samples were Giardia lamblia [19 (6.9%) cases], Entamoeba hystolitica/dispar [6 (2.1%) cases], Trichuris trichiura [6 (2.1%) cases] and Ascaris lumbricoides [5 (1.8%) cases]. Four patients were co-infected with more than 1 intestinal pathogen.
Parasitic results of immigrants children. Number of cases and yield (number of cases/number of test realized) in percentage.
| All patients n (%) | Sub-Saharan Africa n (%) | North Africa n (%) | Latin America n (%) | |
|---|---|---|---|---|
| Intestinal parasitic infections | ||||
| Trichuris trichiura | 6/274 (2.1) | 5/167 (2.9) | 0/64 (0) | 1/43 (2.3) |
| Ascaris lumbricoides | 5/274 (1.8) | 4/167 (2.3) | 0/64 (0) | 1/43 (2.3) |
| Taenia sp. | 1/274 (0.3) | 0/167 (0) | 1/64 (1.6) | 0/43 (0) |
| Hymenolepis nana | 5/274 (1.8) | 1/167 (0.6) | 4/64 (6.2) | 0/43 (0) |
| Endolimax nana | 1/274 (0.3) | 1/167 (0.6) | 0/64 (0) | 0/43 (0) |
| Giardia lamblia | 19/274 (6.9) | 9/167 (5.3) | 8/64 (12.5) | 2/43 (4.6) |
| Entamoeba histolytica/dispar | 6/274 (2.1) | 3/167 (1.7) | 2/64 (3.1) | 1/43 (2.3) |
| Strongyloides stercoralis | ||||
| S. stercoralis detection larvae | 3/274 (0.3) | 1/167 (0.6) | 0/64 (0) | 2/43 (4.6) |
| EIA Strongyloides sp. | 77/341 (22.5) | 64/229 (27.9) | 6/67 (9.0) | 7/45 (15.6) |
| Schistosomaspecies | ||||
| S. haematobium egg in urine | 5/9 (55.5) | 5/9 (55.6) | nd | nd |
| EIA Schistosoma sp. | 42/228 (18.4) | 42/228 (18.4) | nd | nd |
| Filarias species | ||||
| Loa-loa | 2/138 (1.4) | 2/138 (1.4) | nd | nd |
| Mansonella perstans | 9/138 (6.5) | 9/138 (6.5) | nd | nd |
| Microfilaria sp. | 2/138 (1.4) | 2/138 (1.4) | nd | nd |
| Onchocerca volvulus | 1/40 (2.5) | 1/40 (2.5) | nd | nd |
| EIA Filarias sp. | 82/228 (36.0) | 82/228 (36.0) | nd | nd |
| Others | ||||
| EIA Fasciola hepatica | 32/338 (9.4) | 28/228 (12.3) | 3/66 (4.5) | 1/44 (2.3) |
| HAI cisticercosis | 14/309 (4.5) | 13/205 (6.3) | 0/68 (0) | 1/36 (2.8) |
| HAI hidatidosis | 6/316 (1.8) | 4/230 (1.7) | 2/43 (4.7) | 0/43 (0) |
| EIA Trypanosoma cruzi | 1/43 (2.3) | nd | nd | 1/43 (2.3) |
We did not detect differences in the global prevalence of intestinal parasitosis between Sub-Saharan Africa, Northern Africa and Latin America (11.9% vs 17.1% vs 9.3%, respectively) (p=0.6).
The diagnosis of strongyloidiasis was more frequent in minor from Sub-Saharan Africa [64 (27%) cases vs 13 (11%) cases from other areas, OR 2.9 [1.8–5.6], p<0.001].
Logistic regression was used to analyze the length of stay compared to the performance of the stool samples, resulting in an index of β=−0.020 (p=0.07). For each month of stay, the probability of a positive finding in the stool sample decreased by 0.02%.
Microfilarias were detected using the Knott test and skin snips in 13 (9.4%) out of 138 and one (2.5%) out of 40 children from Sub-Saharan Africa, respectively, as shown in Table 2. The ELISA test was positive in 82 (36%) cases.
Schistosomiasis was detected in 42 (18.5%) Sub-Saharan children: five by eggs in urine and 42 only by the serological method. Hematuria was associated with the final diagnosis of schistosomiasis (9 out of 11 patients, p=0.003).
DiscussionParasitic infectious diseases are one of the most frequent causes of illness in child inhabitants in low-income countries.15–17 Therefore, soil-transmitted helminths are considered the second leading cause of mortality in children less than 6 years of age who live in Africa.16
Notwithstanding the importance of health status, few studies have focused on imported parasitic diseases in immigrant minors.18–20 Legal barriers to testing such as the age of consent in children and adolescent, especially when the guardians are non available, may contribute to the lack of studies, and consequently to develop guideline of screening of minors immigrants arrived from areas of high-risk of parasitic infection.
Thus, the aim of our work was studier prospectively in a population of asymptomatic or paucisintomatic immigrants minors the parasitic infections most frequent according its origin area using parasitological and serological methods.
The children and adolescents were referred from different host charities and they were asymptomatic or with unspecified symptoms (usually digestives or skin symptoms). The legal tutors firm the informed consent and the patients were also informed. The subject, only were referred if they accepted voluntarily to be included in the screening. The protocol previously defined consisted in anamnesis and physical examination, examining stool samples, blood and urine samples. We included in the study all patients referred regardless of whether the requested samples collected. This is the reason because the number of samples analyzed was lower than the number of patients included in the study.
Screening for parasitic infections was focused according the origin of the minors. Thus, whereas all patients studied were screened for copro-parasitic and immunodiagnostic tests (detection of antibodies for Strongyloides sp., E. granulosus, cysticercosis and Fasciola hepatica), only children from Sub-Saharan areas were screened for filarial and schistosomal infections. Finally, we also screened minors from Latin America using an EIA for T. cruzi.
Using direct and serological tests, we detected a rate of parasitic infection of 47%; the detection was most frequent in children from Sub-Saharan Africa vs other origins. As reported in previous studies in adult immigrants, the inclusion of serological tests permitted the detection of frequent cases of co-parasitization by two or more parasites.21 We have not found any clear explanation of the higher percentage of parasitosis in children coming from urban area.
Intestinal parasites were detected in nearly 20% of the patients studied. This figure is less than was previously reported in other works, where the overall prevalence of intestinal parasitic diseases reached 75%.18–20,22 This reduced prevalence of intestinal parasitosis in our study may be explained because more than half of the children had not arrived recently (<6 months). As shown in our study and other works, the percentage of intestinal parasitosis decreases according to the length of stay in the host country.23
The most frequent intestinal parasite detected in our patients was G. lamblia, followed by Trichuris trichura and A. lumbricoides. The high percentage of infection by G. lamblia is in agreement with another study that examined 1042 internationally adopted children and detected G. lamblia as the most frequent parasite in the population.24
Intestinal parasites lead to malabsorption and chronic blood loss in children, with long-term effects on their physical (height-weight) and cognitive development. These infections represent a social and economic problem in developing countries.15–17 However, in our environment these infections disappear with time when the cycle of re-infection is stopped due to hygienic and sanitary conditions and rarely cause serious complications.
Strongyloides sp. was the other frequently detected parasite. However, we only detected 3 patients with Strongyloides larvae using an agar-plate culture test. It is obvious that systematic use of this concentration's methods would increase the number of patients with direct diagnosis.25
With respect to immunodiagnostic tests for Strongyloides, in our study we administered an EIA test using a whole antigen of Strongyloides venezuelensis with good sensitivity and specificity previously reported by Machado et al.26 Using this EIA, we detected 77 (22.5%) seropositive patients. This result is in agreement with other studies in adult immigrants using immunodiagnostic methods.21 Diagnosis of this parasitic infection in children is very important because Strongyloides can persist in the host for decades; the use of corticoids or other immunosuppressive drugs by these subjects years afterwards could cause a hyper-infection syndrome with dramatic consequences. These data suggest the need to include Strongyloides screening in all immigrant children coming from endemic areas.27
Furthermore, we performed a screening for filarial and schistosomal parasites in the Sub-Saharan children. Using the Knott test and skin snips we detected filarial infections in 13 and 1 patients, respectively, showing a yield of 10% and 2%. These yields were less than those reported by other authors who used direct methods to detect the percentage of infection (21.9%).19
We also used an EIA based on the whole antigen of Dirofilaria immitis. This test has been used by other authors for the diagnosis of filarial infections.28 Using this EIA, we detected 36.0% seropositivity, which is an increase of three-fold compared to the direct diagnosis method. This result can be explained by cross-reactions with other helminths and by a high percentage of occult filariasis (amicrofilaremic) that in endemic areas, but also in our country, can represent as many as two-thirds of all patients with filariasis.29
In our work, the prevalence of schistosomiasis in children from Sub-Saharan Africa was lower compared to that reported by other studies in adults.21 This discrepancy could be explained by the inclusion of children from Senegal and Equatorial Guinea, which have a lower prevalence of schistosomiasis,30 and the low yield of stools, possibly due to inadequate processing of the samples. The Ritchie test (concentration of eggs by flotation) was not a good method for the detection eggs from trematodes such as Schistosoma sp. and F. hepatica because we obtained lower yield, than using Kato Katz test. Whetham et al. reported similar results.31
The divergence between results obtained from serological and direct diagnostic methods may be associated to a high frequency of past infections in an endemic environment, but also to a low rentability of conventional, time-consuming parasitologic methods, whose success is closely linked to the staff knowledge and experience, especially in countries where most of these parasites were exceptional until recently. This makes advisable the evaluation of molecular methods for a more reliable diagnosis of these parasitic infections in developed countries.
This study had several limitations. First, our work does not represent the general minor immigrant population in Spain in terms of area of origin because the majority is from North Africa and Latin America. Second, the high disease rates in Africa make it more likely that immigrants from this continent will have an elevated rate of disease and therefore be referred to the TMO. Thus, the frequencies of symptoms and diagnoses may be biased by the imbalance in areas of origin of the patients. Third, the collective study is biased because it includes children treated during a specific consultation for tropical diseases. For these reasons, among others, the disease rates found in this study should be analyzed and interpreted with caution before extrapolating them to the general minor immigrant population. Finally, we should note that an important part of the diagnosis of filariasis and strongyloidiasis are serologic and therefore may be due to cross-reactions with other helminth infections or past infections.
Despite these limitations, our study shows interesting data, that can support development of guidelines of diagnosis in population minor immigrant. This guideline will allow us the lower the barrier legal for screening, and consequently an earlier diagnosis and treatment in these un-favored population.
In conclusion, half of the immigrant minors studied presented with parasitic infection, and more than twenty percent harbored multiple parasites. The high infection rates of some parasitic diseases in immigrant children highlight the need to screen for certain infectious diseases in these children according to their country of origin and their length of residence in western countries and to evaluate this population for treatment for helminth infections with antiparasitic drugs upon arrival.
FundingThis work was supported by Proyecto de investigación socio sanitaria Junta Castilla y León (SOCIO673/SA/05/08).
Conflict of interestAll authors declare no potential conflicts of interest.