Prime time is the period of peak evening hours for television viewing. Like so many other parts of language, the term "ready for prime time" has moved from television to society at large, and is taken to mean those things that are proven and ready for general use. The results of two clinical trials in this month's Spanish Journal Enfermedades Infecciosas y Microbiología Clínica make this an opportune moment to reconsider short-course regimens for the treatment of latent tuberculosis infection. Despite the proven efficacy and safety of isoniazid monotherapy, there are growing concerns about the overall effectiveness of isoniazid treatment for latent tuberculosis as a disease control strategy. Some of the limitations of isoniazid treatment for this purpose are inherent in any drug treatment for latent infection, particularly the lack of interest among asymptomatic persons in taking drug therapy for an infection signified only by a skin test reaction or blood test result1. Other generic barriers to initiation of drug therapy are concerns about the need for blood testing1, and the fear of the stigmatizing label of "tuberculosis".
However, the biggest limitation of isoniazid treatment for latent tuberculosis is the duration of therapy required. If it is a challenge to convince asymptomatic persons to start drug therapy, it is very difficult to convince them to complete 6 to 9 months of that therapy. In program settings, completion rates for isoniazid treatment of latent tuberculosis range from marginal to dismal2-4, and greatly limit the overall effectiveness of this intervention. Among HIV-infected persons, there are two additional limitations of isoniazid treatment of latent tuberculosis: the apparent limited durability of protection from active tuberculosis among persons in tuberculosis-endemic areas5 and the lack of efficacy in preventing active disease among anergic patients at high risk of tuberculosis exposure6.
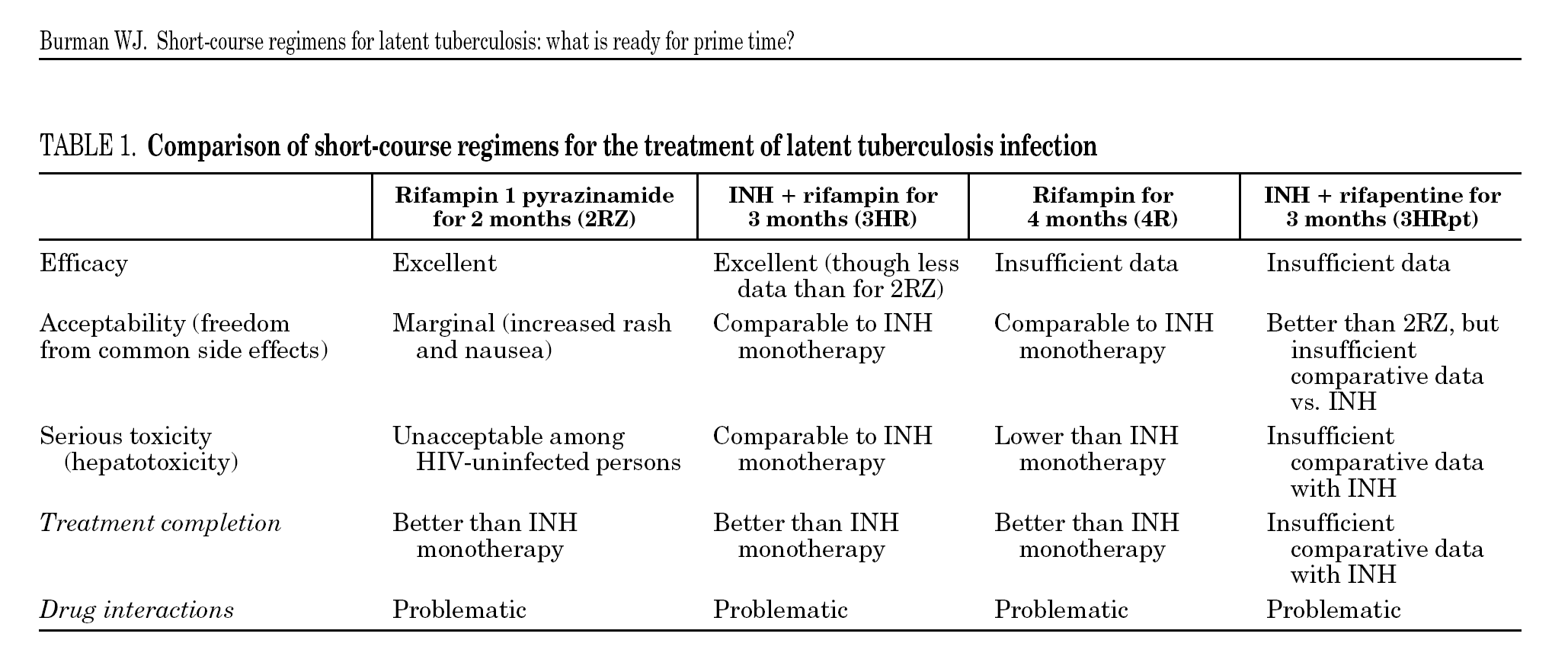
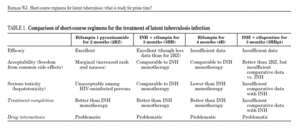
These considerations have led to considerable efforts to develop and evaluate shorter regimens for treating latent tuberculosis. Based on the excellent results of short-course regimens for active tuberculosis, the short-course regimens for latent tuberculosis include one or both of the "sterilizing drugs": rifampin (or a related rifamycin) and pyrazinamide. Animal models of chronic, low-level tuberculosis infection suggest that regimens including a rifamycin and/or pyrazinamide would be effective for latent tuberculosis in humans7. Four short-course regimens have been assessed in humans (table 1). In 2007, after 15 years of clinical trials, which of these regimens are ready for prime time?
Successful treatment of an asymptomatic infection requires antimicrobial potency (efficacy among adherent patients), acceptability (lack of bothersome side effects), lack of serious uncommon side effects, and a duration that promotes treatment completion. An ideal regimen for treatment of latent tuberculosis would also be inexpensive and not have drug-drug interactions with medications commonly used among persons with latent tuberculosis. None of the available regimens satisfy all these conditions, although some may fulfill enough criteria to be considered for routine use in selected clinical situations.
Of the available regimens, the combination of rifampin plus pyrazinamide for 2 months (2RZ) has undergone the most stringent test of efficacy success in preventing active tuberculosis among HIV-infected persons in multiple clinical trials and appears to be at least as potent as 6 to 12 months of isoniazid8. Despite great enthusiasm following initial studies among HIV-infected persons, 2RZ has been essentially abandoned as a regimen for latent tuberculosis because of toxicity among HIV-uninfected persons. In formal clinical trials and programmatic settings, 2RZ was associated with a unacceptable risk of drug-induced hepatitis9,10, including occasional cases of overt liver failure and even death11. Remarkably enough, the excess hepatotoxicity of 2RZ appears to be limited to HIV-uninfected persons. The study by Rivero and colleagues12 comparing 2RZ with 6 months of isoniazid (6H) adds to the substantial body of evidence that 2RZ may not cause excess hepatoxicity among HIV-infected persons13.
Therefore, should 2RZ be used for HIV-infected patients with latent tuberculosis? Despite the lack of excess hepatotoxicity in the four randomized trials of 2RZ vs. isoniazid, there have been cases of severe hepatotoxicity among HIV-infected persons treated with 2RZ11. These cases suggest that the trials were not large enough to detect rare, but serious hepatotoxicity. Furthermore, it is not clear that laboratory monitoring (e.g., hepatic transaminases every 2 weeks) is sufficient to prevent cases of severe hepatoxicity11. While one could argue that there is no evidence of excess risk of severe hepatotoxicity from 2RZ among HIV-infected persons, it is difficult to recommend 2RZ given the emerging data on the activity and tolerability of other short-course regimens.
Following the recognition of hepatotoxicity from 2RZ, there has been increasing interest in regimens including a rifamycin, but not pyrazinamide. The regimen that has received the most extensive evaluation to date is isoniazid plus rifampin for 3 months (3HR). In the only trial powered for a comparison of tuberculosis outcomes, 3HR was as potent as 6H among patients with HIV co-infection14. Indeed, with prolonged follow-up, the 3HR regimen appeared to have a more durable protective effect than did 6H5. Randomized trials by Rivero and colleagues12 and Gerjo and colleagues15 in this month's edition of the Journal, provide additional important data on the 3HR regimen. While not powered for tuberculosis outcomes, these two studies mirror the results of other trials and programmatic reviews in suggesting that the 3HR regimen is as potent as 6H.
Unlike the 2RZ regimen, 3HR appears to be well tolerated among both HIV-infected and HIV-uninfected persons. Martinez and colleagues even raise the question of whether 3HR is safer than 6H, perhaps by limiting the duration of exposure to isoniazi15. However, the reported trend towards excess "hepatoxicity" (44% with 6H vs. 29% with 3HR, P = 0.07) is likely due to the detection of elevated hepatic transaminases of doubtful clinical relevance through routine biochemical monitoring; there was no difference in rates of clinical hepatoxicity. Not surprisingly, in this and other trials, the shorter regimen was associated with a significant improvement in treatment completion (90.2% with 3HR vs. 75.6% with 6H, P = 0.05).
What is the place of the 3HR regimen in current therapy for latent tuberculosis? It would be ideal to have more than one large disease-endpoint trial to more definitively assess the potency of this regimen. However, the data from smaller trials and programmatic reviews provide additional reassurance that 3HR has comparable activity to isoniazid for 6 to 9 months16. Even if somewhat less potent (which seems unlikely), the overall effectiveness of 3HR is likely to be superior to 6-9H simply because of the higher treatment completion rate. The primary limitation of this and other proposed short-course regimens is the inclusion of rifampin, with its problematic propensity for causing clinically significant drug-drug interactions.
Other proposed short-course treatments for latent tuberculosis include rifampin monotherapy (4 months) and once-weekly rifapentine plus isoniazid for 3 months. Rifampin monotherapy appears to be very well-tolerated (perhaps even better tolerated than isoniazid)17 and may be as potent as 6 to 9 months of isoniazid. However, given the serious unintended consequences of rifampin monotherapy for active tuberculosis, it seems doubtful that rifampin alone will make it to prime time. The once-weekly regimen of rifapentine plus isoniazid has the advantages of being both short (3 months) and highly intermittent (requiring just 12 doses), thereby lending itself to directly observed therapy. At present, there are insufficient data on the efficacy of this regimen, though it appears to be relatively well-tolerated18. However, a large randomized trial (n = 8000) comparing 3HRpt to 9H has almost completed enrollment (Tuberculosis Trials Consortium Study 26), and final results of this trial can be expected in 2010.
With these emerging data on short-course regimens, it is time for tuberculosis control programs to evaluate their outcomes with treatment of latent tuberculosis. If high treatment completion rates are not being achieved with 6 to 9 months of isoniazid, particularly among high-risk groups of patients (children, contacts to active cases, HIV-infected persons), the 3HR regimen should receive strong consideration. Combining potency with tolerability and a patient-friendly duration, the 3HR regimen may be a key intervention in improving outcomes of latent tuberculosis treatment it is ready for prime time.
Correspondence: Dr. W.J. Burman.
Denver Public Health.
605 Bannock St. Denver CO USA 80204.
E-mail: bburman@dhha.org
Manuscript received on March 19, 2007; accepted for publication on March 22, 2007.