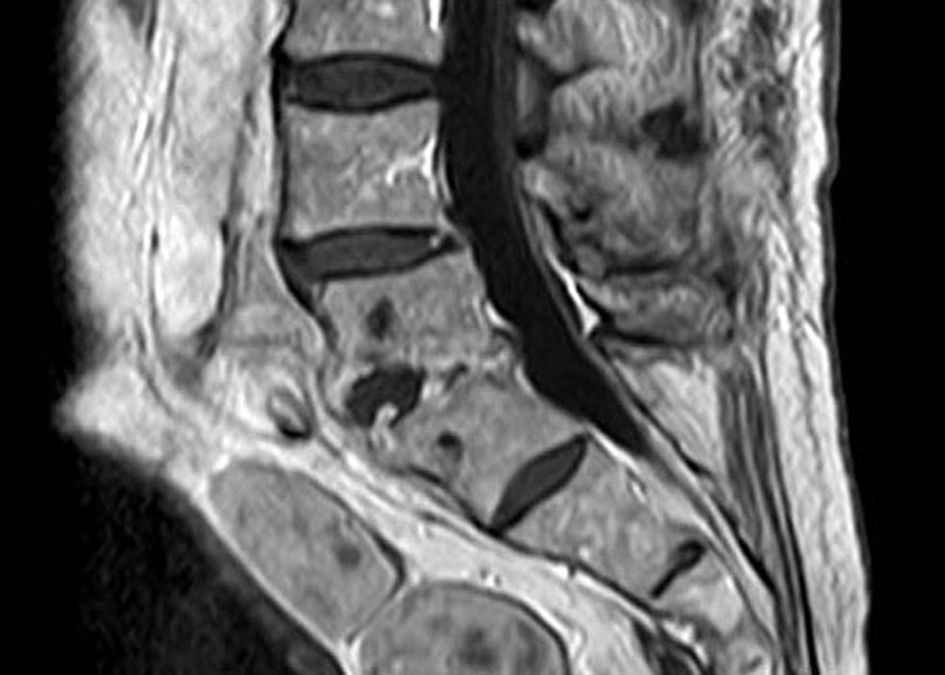
In January 2015, a 79-year-old man visited the emergency room complaining of increasingly severe low lumbar pain, which he had had during the previous 2 weeks. The patient presented leg pain, originating from the buttock region and radiating into the whole thigh, leg and foot. He was apyretic and had lost 18kg. He had no particular antecedents except for a prostatic adenoma. The patient was hospitalized to control pain. Three days after admission to hospital, blood and urine specimens were taken for culture and magnetic resonance imaging (MRI) was performed. MRI revealed increased signal intensity at the L4-L5 position, compatible with a diagnosis of spondylodiscitis at this location (Fig. 1). Blood cultures were negative, although a urine culture yielded growth of Enterococcus faecalis. The patient was initially treated with intravenous ampicillin and gentamicin, assuming that E. faecalis was responsible for the discitis. Twelve days after admission to hospital, disk fluid were obtained by aspiration. Gram staining of the substance obtained from the aspiration revealed abundant leukocytes with a few gram-positive cocci. After 24h, cultures performed on blood agar (bioMérieux, Marcy l’Étoile, France) yielded small alpha-hemolytic colonies. The isolate was identified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry system (Bruker Daltonics, Leipzig, Germany) as Aerococcus urinae. 16S rDNA PCR revealed the presence of A. urinae in this sample, confirming a diagnosis of A. urinae spondylodiscitis. After isolation of A. urinae, gentamicin was discontinued and the patient remained with intravenous ampicillin for 3 weeks. Susceptibility was determined using Clinical Laboratory Standards Institute (CLSI) Aerococcus spp. criteria.1 The following minimum inhibitory concentrations (MIC) were obtained: penicillin, 0.015mg/L; ampicillin, 0.125mg/L; cefotaxime, 0.094mg/L; levofloxacin, 0.5mg/L; vancomycin, 0.5mg/L; erythromycin 1mg/L; and clindamycin, 2mg/L. The isolate was susceptible to all antimicrobials tested, except to erythromycin and clindamycin. No interpretive criteria for aerococci had been set for erythromycin and clindamycin at that time, so CLSI viridans group streptococci interpretive criteria was used.2 During antibiotic therapy, the patient obtained relief from the back pain. Five weeks after admission to hospital, the patient was discharged with oral therapy of amoxicillin (5 months). The follow-up MRI 5 months after discharge showed reduction of the space between the L4-L5 vertebrae and loss of disk space height, but no sign of infection. The patient was followed up during a year and the resolution of the disease was complete, with no severe functional sequelae or neurological deficit. He was autonomous and independent.
Aerococcus species are Gram-positive cocci that grow predominantly in tetrads and clusters, but unlike staphylococci, they are catalase-negative.3 When grown on blood agar media in 5% CO2, A. urinae forms small, alpha-hemolytic colonies that may be confused with viridans group streptococci or enterococci. Due to these similarities, these organisms have often been misidentified, leading to the causes of human infection being underestimated.4 Before the introduction of new methods of species identification, only occasional clinical case reports were noted. With the introduction of MALDI-TOF into routine laboratory analyses, however, awareness of aerococci as human pathogens has increased.5 When A. urinae was isolated from urine culture, the proportion of patients with symptoms indicating urinary tract infection (UTI) and without symptoms (classified as colonized) was almost the same.6 Hence, the isolation of A. urinae from urine in symptomatic patients does not formally prove that this is the pathogen causing UTI. However, a UTI focus is frequently suspected in invasive A. urinae infections.5 The most common presentation of invasive A. urinae infection is endocarditis, with a relatively favorable prognosis.7 Patients with invasive A. urinae infections are typically older men with underlying urinary tract disease, many with a urinary tract catheter.5,7 To date, two spondylodiscitis cases caused by A. urinae have been reported, probably associated with endocarditis, and the organism was recovered from blood samples.8,9 Although grampositive vertebral infections are frequently associated with endocarditis when a routinary echocardiographic study is performed,10 the absence of bacteriemia suggest that this case is the first isolation of A. urinae in disk fluid not associated with endocarditis.
Although some studies suggest favorable outcomes with 4–6 weeks treatment course in uncomplicated vertebral osteomyelitis,10 we treated our patient for a long period, because there are no treatment studies on aerococcal infections.4 The β-lactam-aminoglycoside combination is not entirely clear because, although this combination has been shown to be synergistic in vitro for A. urinae isolates, a recent study could only demonstrate this synergy in a few cases.4 Hence is importance to distinguish Aerococcus from Enterococcus in these infections, because bone enterococcal infections are difficult to cure and require combination therapy, including ampicillin–aminoglycoside.
The introduction of MALDI-TOF as a routine part of laboratory work will be very helpful for the identification of A. urinae in clinical samples. A. urinae is only occasionally isolated in clinical specimens, hence this clinical report of spondylodiscitis due to A. urinae isolated from disk fluid helps reveal the pathogenic potential of this bacterium as a cause of bone infection.
Conflict of interestThe authors declare no conflict of interest.