To analyse clinical and epidemiological characteristics of immigrant patients diagnosed with strongyloidiasis in our area.
MethodsAn analyse was performed on patients with strongyloidiasis seen in the Tropical Medicine Unit of the “Hospital de Poniente” in Almeria (Spain), from April 2004 to May 2012.
ResultsA total of 320 patients were diagnosed with Strongyloides stercoralis infection, and 284 out of 314 patients (90.4%) had a positive specific serology. Forty-two percent of the patients reported symptoms and 45% had eosinophilia. The serological results were monitored in some of the patients, confirming a loss of antibodies in all 20 patients studied.
ConclusionsStrongyloidiasis is a parasitic disease increasingly diagnosed in developed countries due to increased migratory flows from endemic areas. Often being asymptomatic, its diagnosis and treatment may prevent fatal outcomes, especially in immunocompromised patients.
Analizar las características clínicas y epidemiológicas de los pacientes inmigrantes diagnosticados de strongyloidiasis en nuestra área.
MétodosSe analizaron retrospectivamente los pacientes con strongyloidiasis que acudieron a la Unidad de Medicina Tropical del Hospital de Poniente de Almería (España), entre abril de 2004 mayo de 2012.
Resultados320 pacientes han sido diagnosticados con infección por S. stercoralis, 284/314 pacientes (90,4%) tenían una serología específica positiva. 42,3% de los pacientes presentaron síntomas y el 45% de los pacientes tenían eosinofilia. La monitorización del tratamiento confirmó la pérdida de anticuerpos en los 20 pacientes estudiados.
ConclusionesLa estrongiloidiasis es una parasitosis diagnosticada cada vez con más frecuencia en países desarrollados debido al aumento de los movimientos migratorios procedentes de zonas endémicas. Siendo a menudo asintomática, su diagnóstico y tratamiento pueden prevenir resultados fatales.
Strongyloidiasis is a helminthic infection caused by Strongyloides stercoralis, a nematode ubiquitous in tropical and subtropical countries including Africa, Southeast Asia, and Latin-America1 and occasionally reported in temperate countries, including Spain.2,3 The estimated prevalence of strongyloidiasis is around 50–100 million infections worldwide, although the accuracy of these estimates is uncertain due to poor sensitivity of screening methods.2,4
S. stercoralis is unique in its ability to replicate in the human host, permitting on-going cycles of auto-infection that can persist for decades without further exposure to exogenous infection.2
Disease spectrum for strongyloidiasis includes acute infection with Loeffler's syndrome, chronic intestinal infection, asymptomatic auto-infection, symptomatic auto-infection, and hyper-infection syndrome with massive dissemination through the bloodstream,5,6 particularly in patients with HTLV-1 or HIV infection, or on steroid medication and other immunosuppressive drugs, such as organ transplant recipients.5–7
Several diagnostic methods have been compared to detect the presence of S. stercoralis including stool examination, stool culture, polymerase chain reaction (PCR) and specific antibodies detection.8
As immigrant population and international travels continue to increase, imported tropical diseases are becoming more frequent in developed countries. In order to preclude the re-emergence of strongyloidiasis in such areas as the Mediterranean one, where transmission is rare but possible, screening may be recommended. In contrast, to prevent hyper-infection syndromes among immunosuppressed immigrants or previous to the administration of immunosuppressive therapies (i.e. pre-transplantation), early diagnosis and treatment is mandatory.
The objective of our study is to analyse the clinical and epidemiological characteristics of migrants patients diagnosed with strongyloidiasis in our area.
MethodsWe undertook an observational study analysing retrospectively the epidemiological and clinical characteristics, microbiological findings, and outcome of those patients with strongyloidiasis who attended the Tropical Medicine Unit of the Poniente Hospital in Almeria (Spain), from April 2004 to May 2012. This Unit serves as the health care reference centre for an area of nearly 300,000 inhabitants, where close to 30% of the population are migrants from low rent countries.
All migrants from endemic areas referred to the clinic were studied in order to rule out S. stercoralis infection by means of parasite search in stool smears from 3 stool samples (direct stool examination and/or faeces larvae culture) and serology, although not every patient was studied with all three techniques. Parasite stool culture was undertaken following each patient's attending physician criteria. Diagnosis of strongyloidiasis was considered when any of these three tests yielded a positive result.
Diagnosis of strongyloidiasis was made by detection of rhabditiform larvae in stool (3 stool samples examined by light microscopy using formalin-ethyl acetate concentration techniques), detection of forked tailed filariform larvae in a culture with charcoal Dancescu, or detection of specific Strongyloides IgG antibodies (ELISA kit PALEX Medical SA, Ref EIA-4208, DRG Diagnostics, Germany).
Demographic data were obtained from patients’ clinical history and statistical analysis was performed using SSPS 15.0 program, using mean and standard deviation as central tendency and dispersion measurements respectively.
ResultsDuring the study period, 1384 immigrant patients have been evaluated in this Unit, of which 320 (23%) have been diagnosed with S. stercoralis infection. Most patients attended in our Tropical Medicine Unit were referred from Primary Health Care Institutions (67%). Patients’ regions of origin were sub-Saharan Africa (89%), Maghreb (4.7%) and Latin America (6.3%). HIV infected patients were excluded from the study and attended in a specific clinic.
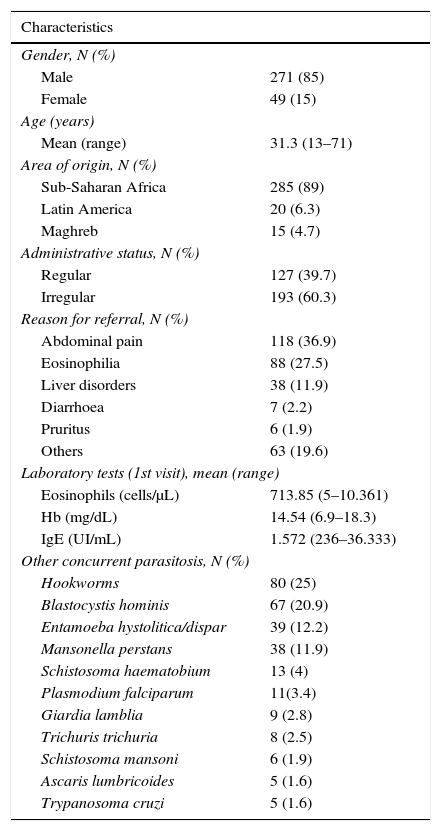
Medical records of all patients were reviewed and epidemiological and clinical and analytical data registered. Data are shown in Table 1. One hundred and thirty-one (42.3%) patients reported symptoms, mainly gastrointestinal complaints such as abdominal pain (38.4%) or diarrhoea (2.3%), and pruritus (2%).
Characteristics of the patients (N=320).
| Characteristics | |
|---|---|
| Gender, N (%) | |
| Male | 271 (85) |
| Female | 49 (15) |
| Age (years) | |
| Mean (range) | 31.3 (13–71) |
| Area of origin, N (%) | |
| Sub-Saharan Africa | 285 (89) |
| Latin America | 20 (6.3) |
| Maghreb | 15 (4.7) |
| Administrative status, N (%) | |
| Regular | 127 (39.7) |
| Irregular | 193 (60.3) |
| Reason for referral, N (%) | |
| Abdominal pain | 118 (36.9) |
| Eosinophilia | 88 (27.5) |
| Liver disorders | 38 (11.9) |
| Diarrhoea | 7 (2.2) |
| Pruritus | 6 (1.9) |
| Others | 63 (19.6) |
| Laboratory tests (1st visit), mean (range) | |
| Eosinophils (cells/μL) | 713.85 (5–10.361) |
| Hb (mg/dL) | 14.54 (6.9–18.3) |
| IgE (UI/mL) | 1.572 (236–36.333) |
| Other concurrent parasitosis, N (%) | |
| Hookworms | 80 (25) |
| Blastocystis hominis | 67 (20.9) |
| Entamoeba hystolitica/dispar | 39 (12.2) |
| Mansonella perstans | 38 (11.9) |
| Schistosoma haematobium | 13 (4) |
| Plasmodium falciparum | 11(3.4) |
| Giardia lamblia | 9 (2.8) |
| Trichuris trichuria | 8 (2.5) |
| Schistosoma mansoni | 6 (1.9) |
| Ascaris lumbricoides | 5 (1.6) |
| Trypanosoma cruzi | 5 (1.6) |
Forty-five percent (144) of patients with strongyloidiasis had eosinophilia (≥500cells/μL) at first visit.
Mean absolute eosinophil count was 713.85cells/μL (SD 842.5), mean serum IgE 1572UI/mL (SD 2981.5) and mean haemoglobin 14.5mg/dL (SD 1.74).
Other parasitises was frequent, with a total of 142 (44%) patients infected with other parasites besides Strongyloides. Table 1 shows concurrent parasitosis total figures.
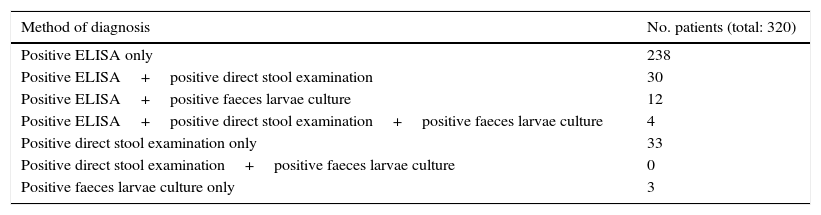
The results of the various diagnostic tests performed are shown in Table 2.
Diagnostic tests used for Strongyloides infection diagnosis.
| Method of diagnosis | No. patients (total: 320) |
|---|---|
| Positive ELISA only | 238 |
| Positive ELISA+positive direct stool examination | 30 |
| Positive ELISA+positive faeces larvae culture | 12 |
| Positive ELISA+positive direct stool examination+positive faeces larvae culture | 4 |
| Positive direct stool examination only | 33 |
| Positive direct stool examination+positive faeces larvae culture | 0 |
| Positive faeces larvae culture only | 3 |
Serologic test (ELISA) was performed in 314 patients (98.1%). Results were positive in 284 patients (90.4%).
Direct stool examination was performed in 293 patients (91.6%). Results were positive in 67 patients (22.9%).
Faeces larvae culture was performed in 51 patients (15.9%). Results were positive in 19 patients (37.3%).
Human T-lymphotropic virus type 1 (HTLV-1) infection was tested in six patients with persistent disease despite a correct treatment, being positive in one of them.
Standard treatment was Ivermectin, 200μg/kg/day divided in 2 doses, for 2 consecutive days.
For patients with persistent infection, including that one patient with HTLV-1 infection, same treatment was re-administered. Patients were considered cured based on the disappearance of symptoms and/or eosinophilia. In those patients in whom larvae were visualised in stool samples or with a positive coproculture, a negative result of these tests was found in later controls. During last year, serological response to treatment was monitored in 20 patients (6.25%), confirming significant falling of even negativization of antibodies in 19 of them.
DiscussionStrongyloidiasis is a major global health challenge underestimated in many countries. It remains as an important helminthic disease due to increases in travel and migration from endemic countries.2,3 Twenty-two percent of all patients treated in our Unit have been diagnosed with strongyloidiasis, with a mean time of residence in Spain of 48.11 months (range 1–256), many having been living in Spain for years, reflecting the potential of S. stercoralis for causing persistent infection and becoming an emerging disease.6
Diagnosis of S. stercoralis may be missed when not specifically sought and it is often delayed due to patients presenting with non-specific gastrointestinal complaints. Only 42.3% of our patients reported symptoms associated with chronic infection, mainly abdominal pain (38.4%). Peripheral eosinophilia may often be the first hint of a parasitic infection,5 however, less than 50% of our patients had eosinophilia. Eosinophilia is an inconstant finding in S. stercoralis infection and may fluctuate in different degrees.4 Besides, eosinophilia is an unspecific finding as may be due to other helminthic parasitosis.
Due to a low parasite load and irregular larval output, multiple stool sample tests are required for accurate diagnosis (being a single sample positive in only 30–50% of cases).6,9 In our study, S. stercoralis larvae in stool samples were found in only 22.9% of patients. Multiple repeated stool studies and other techniques are needed to improve sensitivity but these are more laborious, time consuming and expensive.9,10
Owing to the technical difficulties for detecting Strongyloides larvae in stool, serologic tests using crude parasite antigens have been increasingly used for diagnosis, with a reported sensitivity of 83–93%. In our study, 93.4% of the patients had positive serologic results, although 33 patients, in whom larvae were detected in faeces, had a negative serology. On the other hand, specificity of serology is dubious when used among samples of polyparasited individuals, like in our study, because false positive results due to cross-reactions with other nemathelminthes infections might occur.
Recently, it has been advocated that serological studies should be done to assure clearance of infection at 6 and 12 months1 after treatment. Although there is no test of cure currently available, IgG antibody levels decrease markedly within 6 months of successful treatment, and DNA-based diagnosis have lately been shown to hold promise as a proof-of-cure technique.3,10
Throughout the last year, we have also evaluated the response to treatment by serological monitoring, confirming loss of antibodies in all 20 patients studied.
Our study has some limitations. The sample comprised mainly patients from sub-Saharan Africa, raising the question about the possibility of our results not being valid for migrants coming from other geographic areas. Second, the above mention lack of specificity of serologic tests when used among polyparasited individuals; in our series, polyparasited patients comprised 44% of the patients, so false positive cross-reactions should be taken into account. Third, not all three diagnostic techniques – direct stool examination, faeces larvae culture, and specific serology – were performed in every patient. If such were the case, the number of patients diagnosed with Strongyloides infection would surely be greater and we could also had been able to compare diagnostic sensitivity between the three different techniques.
ConclusionStrongyloidiasis may be a silent infection or present with vague symptoms and no specific laboratory findings. In our study, we have searched for Strongyloides infection in all migrants attended at our Unit during a period of eight years, irrespective of the reason they consulted for, finding a significant 23% of migrants being infected by S. stercoralis. Out of these 320 infected patients, only 42.3% reported symptoms related to strongyloidiasis, such as gastrointestinal complaints, and less than 50% showed eosinophilia in the first visit blood test.
Health professionals in developed countries who care for patients from endemic areas, or for patients who have travelled to these areas, must therefore, try to rule out the presence of Strongyloides infection, especially in patients presenting with eosinophilia or unspecific gastrointestinal or pulmonary symptoms and mandatorily, in all HIV infected patients or patients receiving corticosteroid therapy, organ transplantation, or any other type of immunosuppression.
Conflict of interestThe authors declare no conflict of interest.