Currently, an increasing impact of some arboviruses has been observed in Europe, mainly Dengue (DENV), Chikungunya (CHIKV), Zika (ZIKV), West Nile (WNV), and Crimean-Congo hemorrhagic fever (CCHFV) analyzed through a One Health perspective that considers their expansion across the continent. Arboviruses are primarily transmitted by vectors such as mosquitoes and ticks, with human activities and climate change playing crucial roles in their spread. The review highlights the ecological and epidemiological aspects of arboviruses, emphasizing the roles of diverse hosts and reservoirs, including humans, animals, and vectors, in their life cycles. The influence of climate change on the ecology of the vector, which potentially favors the arbovirus transmission, is also reviewed. Focusing on diagnosis, prevention and in the absence of specific treatments, the importance of understanding vector–host interactions and environmental impacts to develop effective control and prevention strategies is emphasized. Ongoing research on vaccines and therapies is crucial to mitigate the public health impact of these diseases.
Actualmente se ha observado un impacto creciente de algunos arbovirus en Europa, principalmente, dengue (DENV), chikungunya (CHIKV), zika (ZIKV), virus de la fiebre del Nilo Occidental (WNV) y el virus de la fiebre hemorrágica de Crimea-Congo (CCHFV) analizando su expansión por el continente desde una perspectiva del concepto «Una Salud». Los arbovirus se transmiten principalmente por vectores como mosquitos y garrapatas, teniendo las actividades humanas y el cambio climático una influencia crucial en su propagación. Esta revisión destaca los aspectos ecológicos y epidemiológicos de los arbovirus, enfatizando la importancia de huéspedes y reservorios, incluyendo humanos, animales y vectores, en sus ciclos. También se revisa la influencia del cambio climático en la ecología del vector, favoreciendo la transmisión de estos virus. Centrándose en el diagnóstico, la prevención y ante la ausencia de tratamientos específicos, se subraya la importancia de comprender las interacciones vectoriales y los impactos ambientales para desarrollar estrategias efectivas de control. La investigación en curso sobre vacunas y terapias es crucial para mitigar el impacto en la salud pública de estas enfermedades.
Arthropod-borne viruses (arboviruses) are RNA viruses with a life cycle that requires both a host and a vector: transmission is preceded by a biological replication in an arthropod vector and these viruses generally circulate among wild animals. More than 130 arboviruses are known to cause human disease. Many outbreaks have been registered in Europe during the last years. Climate change, deforestation, traveling abroad and the increase in vector numbers are the main causes of the increase in outbreaks.
Dengue (DENV) and Chikungunya (CHIKV) are among the most diagnosed arboviral infections in travelers, with projections showing an increase in their prevalence. DENV has rapidly spread globally, with its incidence increasing about 30-fold over the last fifty years. CHIKV now affects 45 countries across all continents except Antarctica. West Nile virus (WNV) outbreaks in birds, humans, and livestock have occurred in multiple areas in Europe and have had a significant impact on animal and human health associated with severe cases of neuroinvasive diseases such as meningitis and encephalitis. It is also the predominant cause of arboviral neuroinvasive disease in North America. Additionally, since 2016, the Zika (ZIKV) has become a significant public health concern for European travelers.1,2
Since its initial detection in the 1950s in Trinidad and Tobago, the incidence of Oropouche virus (OROV) has increased over the past decades, predominantly from rural and forest areas in Brazil, Peru, Ecuador, Argentina, Bolivia, Panama, Colombia, and Venezuela. OROV fever is generally mild and self-limiting as a febrile illness, and typically biphasic, but most cases are indistinguishable from common pathogens endemic to the Americas. Severe clinical presentations, which are rare, could involve the central nervous system and result in aseptic meningoencephalitis. Culicoides paraensis is considered the main OROV vector.3
The emergence and re-emergence of the Phlebovirus genus (Bunyaviridae family) has also gained prominence in recent years. Infection caused by Phlebovirus clinically causes the common symptoms of fever and headache, and rarely presents as meningitis or meningoencephalitis. This infection is known by its symptoms as phlebotomus fever. In the Old World the viruses of this genus have been described in Central Asia, Southern Europe and Africa, specifically in Portugal, Spain, France, Italy, Yugoslavia, Cyprus, Turkey, Iran, India, Pakistan, Greece, Egypt, Finland, Bangladesh, Sudan, Nigeria, Ethiopia, Senegal, Central African Republic, among others. In the New World the presence of these viruses has been described in Panama, Guatemala, Colombia, Brazil, the United States, French Guiana, Trinidad and possibly in Peru.4
Viruses of the Phlebovirus genus, transmitted by diptera of the Psychodidae family, are a cause of a self-limited febrile syndrome during the summer–autumn in Mediterranean countries. Two sand fly-borne phleboviruses in the Old World were historically associated with cases of sandfly fever: Sicily virus (SICV) and Naples virus (NAPV), which is still a tentative species. SICV and NAPV are both responsible for sandfly fever, a self-limiting but incapacitating febrile illness. Toscana virus (TOSV), discovered in 1971, was incriminated as causing central and peripheral nervous system infections in 1983. TOSV can cause aseptic meningitis and meningoencephalitis, as well as several other manifestations affecting the central and peripheral nervous system. These viruses are transmitted via bites of Phlebotomus spp. sand flies.5
In Spain, the presence of the TOSV, Granada, NAPV, Sicilia, Arbia and Arrabida-like viruses has been detected. The widespread presence of vectors of the Phlebotomus genus, especially Phlebotomus perniciosus, in which several of these viruses have been detected, makes it very likely that infections will appear regularly in humans in Spain with a moderate risk for the TOSV and a low risk for the others, in areas with greater vector activity.6
Arboviruses can also be transmitted by tick bites. Tick-borne diseases mainly occur in tropical and also in subtropical areas. The prevalence of these diseases is increasing, triggered by globalization, by the impact of climate change and possibly also due to changes in human behavior, demographics, climate, and land utilization, resulting in the emergence and re-emergence of zoonotic infectious diseases. It should be noted that in the Americas and other regions of the world, such as Europe, ticks are the main vectors for transmission to animals and humans. Ticks transmit several viral agents, called tick-borne viruses (TBV), such as tick-borne encephalitis virus (TBEV) and Crimean-Congo hemorrhagic fever virus (CCHFV), which have re-emerged in multiple areas of the world. Since the first identification of CCHFV in ticks and the description of the first human cases of CCHFV in Spain in 2016, its epidemiological importance has increased.7
This review will focus mainly on the most significant arboviral diseases with the greatest epidemiological significance.
Understanding the expansion of arboviruses in Europe: a one health perspectiveThe risk of transmission of arboviruses within a region primarily depends on the abundance of competent vectors, the presence of amplifying hosts, the vector–host contact rates, and climatic conditions.8,9 In Europe, several arboviruses that may cause disease in humans, such as CCHFV, TBEV, TOSV, and WNV, are endemic and cause sporadic infections and outbreaks during transmission seasons. However, the emergence of autochthonous cases of previously imported arboviruses, like DENV and CHIKV, highlights the region's increasing vulnerability to these pathogens. Social factors, such as increased population movements, and environmental factors, including rising temperatures, deforestation, and urbanization, facilitate the spread of these infections, leading to outbreaks in previously non-endemic areas.
Hosts and reservoirsIn general, arboviruses exhibit complex transmission cycles involving multiple reservoir host species necessary for the virus's maintenance and replication. This complexity contrasts with arboviruses like DENV, which appears to infect only primates. DENV, ZIKV and CHIKV are transmitted by Aedes spp. mosquitoes, with humans representing the amplifying host. However, ZIKV and CHIKV also circulate in sylvatic cycles in Africa and Asia involving non-human primates and wild Aedes spp. mosquito vectors. Additionally, ZIKV has been observed to replicate in reptiles and amphibians, while rodents and bats may also serve as amplifying hosts of CHIKV, although their significance is less well-established compared to primates.1,8–10
When exploring the maintenance of CHIKV in the environment, the potential role of ectothermic vertebrates as reservoirs or hosts has been assessed through serological surveys. These studies revealed the presence of antibodies against various arboviruses in sera from turtles, snakes, lizards, and frogs. Although viremia was detected, it was not associated with disease. However, it remains unclear whether these reptiles achieve viremia levels sufficient to subsequently infect mosquitoes upon biting. Furthermore, several species of domestic and wild animals, including birds, mesocarnivorous mammals, lagomorphs, and members of the armadillo family, do not appear to be competent hosts for CHIKV in nature. As a result, CHIKV does not seem to pose a significant threat to livestock, birds, and wild mammals.9,10
Humans are the suspected link between the sylvatic and urban OROV transmission cycles since the mosquito C. paraensis is present in both urban and rural settings. OROV antibodies have been found in wild birds, sloths, non-human primates, and rodents, but the role of other animals as amplifying hosts requires further investigation. However, the virus’ transmission cycle is poorly understood.3
WNV, persist in nature through complex transmission cycles that involve various Culex spp. mosquito vectors and avian amplifying hosts, following a mosquito–bird–mosquito transmission route. The transmission cycle starts when these mosquitoes feed on susceptible birds, leading to a high virus concentration in the host, which facilitates further spread to uninfected mosquitoes. In this cycle, birds act both as natural reservoirs and amplifying hosts for WNV. Some bird species exhibit mild or no symptoms and gain permanent immunity, while members of the Corvidae family, such as crows, often suffer significant mortality during outbreaks. These fatalities serve as important sentinel indicators for monitoring the virus. Eventually, the virus may spill over to other vertebrates via “bridging” mosquito species, enhanced by environmental factors like strategic location along major migratory bird routes, wetlands, or high density of capable mosquito vectors. In temperate climates, the virus may persist in adult mosquitoes during winter, ensuring its continuity until the next transmission season.11 In addition to birds, naturally acquired WNV infection has been detected in various reptiles and mammals, with most mammals considered incidental hosts due to their low and brief viremia levels, insufficient to infect competent vectors. Dogs play a significant role due to their interactions with ecosystems where vectors and WNV transmission is prevalent, including wetlands, forests, hunting areas and agricultural fields. Globally, dogs’ seroprevalence rates for WNV range from 4% to 37%, with hunting dogs showing higher seropositivity than domestic ones, possibly due to their exposure to infected prey. This suggests that dogs could serve as effective sentinels for WNV surveillance, as evidenced by their higher seropositivity rates compared to humans in the same regions. Additionally, dogs are common hosts for Culex spp. mosquitoes and ticks of the Ixodidae family.11 In Spain, the seroprevalence in various wildlife species, including ruminants, wild boar, red foxes and clinically healthy horses, ranges from 4% to 20%. Despite these findings, only birds and horses are considered significant in the enzootic cycle of WNV. Humans and horses develop symptomatic infections but do not significantly contribute to viral amplification due to the short duration and low titer of their viremia. Additionally, WNV has been isolated from both hard and soft ticks, although they are not significant epidemic/epizootic vectors.11,12
Other arboviruses, such as CCHFV, are found across various vertebrate and arthropod species, circulating through a tick-vertebrate-tick enzootic cycle.13 The main hosts of the virus include cows, sheep, goats, horses, and donkeys. Many mammals, both wild and domestic, experience transient viremias that do not generally result in disease symptoms but can produce detectable antibodies. However, these antibodies often do not correlate with viremias strong enough to infect feeding ticks, thus indicating that mammals are not long-term reservoirs for CCHFV and seroprevalence studies should be interpreted with caution. Instead, ticks sustain the virus across all their developmental stages and through transovarial transmission, serving as true reservoirs and maintaining the virus in nature. Vertebrates merely provide the necessary blood meals and act as potential amplification hosts during brief periods of viremia that can still initiate efficient transmission routes. Most avian species, apart from ostriches, seem resistant to the virus; ostriches, however, develop significant viremia and have been linked to human CCHFV cases. Although the main transmission route is by tick bite (Hyalomma spp.), humans can acquire the illness by direct contact with biological fluids of infected people, while skinning infected animal skin or through nosocomial transmission.12,14
VectorsA comprehensive understanding of zoonotic transmission of pathogens requires an examination of the three primary mechanisms through which infection is conveyed between humans and animals. The initial mechanism is direct transmission, whereby a vector, enzootic or bridge, transmits the virus from an enzootic host to humans. The second mechanism involves amplification within domestic animals, which then transmit the virus to humans. However, humans typically do not develop a significant viremia to sustain further transmission. The third mechanism shifts from an enzootic cycle to a human–mosquito–human cycle, particularly in urban areas, where humans act as amplification hosts and anthropophilic vectors facilitate transmission.8 These findings underscore the importance of temperature, precipitation, and relative humidity in their epidemiological impact, although geographic location influences on how these changes may be experienced even within the same country.2
Mosquito-borne diseasesMosquitoes are crucial vectors for many arboviruses of significant epidemiological concern in Europe. Aedes aegypti and Aedes albopictus are key vectors for ZIKV, DENV, and CHIKV, and demonstrate distinct characteristics influencing transmission cycles.2A. albopictus has notably expanded its presence from 8 EU/EEA countries affecting 114 regions in 2013, to 13 countries encompassing 337 regions by 2023.15 This mosquito species has a lower infection threshold compared to A. aegypti when feeding on a viremic host, with a lifespan generally around one month but varying due to factors like gender, insecticide resistance, and environmental conditions.1,10 Other mosquito species, such as Culex pipiens, are significant vectors for arboviruses like WNV or Usutu virus (USUV) and are found in all European countries except Iceland and Faroe Island.16 This mosquito thrives in different ecological niches compared to Aedes spp. and has an unique behavior that influences transmission cycles. For instance, Culex spp. mosquitoes are primarily active during dusk and dawn while the activity of Aedes spp. is both diurnal and crepuscular.17
The detection of OROV in an Ecuadorian coastal region lacking Culex paraensis suggests that alternative vectors may be involved in OROV transmission. Although the presence of Culex quinquefasciatus was suspected in Brazil and French Guiana, it shows low experimental transmission efficiency. A. aegypti and A. albopictus, key DENV vectors, are ineffective for OROV. However, mosquitoes like Coquillettidia venezuelensis, Aedes serratus, Psorophora cingulate, and Haemagogus tropicalis have been found naturally infected, indicating their potential roles in transmission.3
Climatic elements crucially modify the ecology of these vectors, enhancing mosquito flight range, shortening viral incubation periods, and extending the arboviral season in endemic areas while also facilitating their spread into new regions.1,10 Rising temperatures accelerate mosquito development and reduce virus incubation time, increasing transmission risk. Conversely, extreme temperatures might decrease mosquito survivability.2 Increased precipitation contributes to vector population growth by generating breeding grounds in standing water. The warming trend in Europe, characterized by more frequent and intense heatwaves, and longer summers, is promoting the proliferation of invasive mosquito species and increasing the potential for outbreaks. These climatic changes are critical in shaping the future distribution and impact of these vectors on public health. Projections indicate that the range of A. aegypti will expand toward the poles and extend transmission seasons, increasing the probability of outbreaks.18
Tick-borne diseaseTicks, particularly species such as Rhipicephalus spp., Hyalomma spp., Ixodes spp., and Dermacentor spp., play crucial roles in the transmission of arboviruses like CCHFV or TBEV. The rate of CCHFV-infected ticks ranges from 2% to 4.8% according to different studies carried out in different European countries. Estimates of TBEV prevalence in Ixodes ricinus published so far refer to Southwestern Sweden and range from 0.10% to 0.42%.19,20Rhipicephalus spp. ticks typically stay with one large mammal host through all life stages, whereas Hyalomma spp. ticks transition from small to large mammals from larval to adult stages. In contrast, genera like Ixodes and Dermacentor detach and reattach to different hosts at each life stage. Ticks exhibit limited mobility in the absence of a vertebrate host. However, the capacity of these arthropods to disperse over vast distances is contingent upon their transportation by their vertebrate hosts, particularly migratory birds and ungulates. Hyalomma spp. is present in Southern and Eastern Europe and I. ricinus covers a wide geographic region including Scandinavia, British Isles, central Europe, France, Spain, Italy, the Balkans, East Europe and North Africa. The distribution of these ticks has changed in several countries in recent years, with I. ricinus being found at higher altitudes in Bosnia and Herzegovina and the Czech Republic. Climate variability has a significant impact on tick behavior and distribution. Warmer temperatures extend the active seasons of ticks and alter their life cycle dynamics, spreading these vectors to higher altitudes and more northern latitudes. Climate change, together with changes in land cover and land use, changes in the distribution of tick hosts, and human-induced changes in the environment, have a direct impact on the prevalence and spread of tick-borne diseases. These changes are critical in determining the future epidemiology of tick-borne arboviruses in Europe.12,21,22
Ecosystems and human interventionEcosystem transformations significantly impact the vectors’ geographical distribution and enzootic/zoonotic transmission cycles. For instance, areas with extensive forest cover have been negatively associated with the population density of C. pipiens. In contrast, suburban and agricultural areas have higher populations of these mosquitoes, increasing the risk of viral transmission, while highly urbanized areas are at lower risk because they have fewer suitable habitats.2 Some studies have observed a linear increase in DENV incidence with rising average temperatures, ambient relative humidity and precipitation. However, excessive rainfall can potentially reduce outbreak probability by washing mosquito eggs from their habitats. Historically, the emergence of certain arboviral diseases like CCHFV has been linked to climatic factors, such as the unusually high tick populations during the spring and early summer of 1944.2,12
Human behavior also contributes significantly to the current spread of these arboviral diseases. Factors such as globalization, trade and transport, demographic changes, urbanization, and the movement of populations between rural and urban areas—often accelerated by war or natural disasters—play crucial roles. Vectors also interact with population movements: infected travelers returning to areas with competent mosquitoes can lead to local clusters or outbreaks. In Spain, the number of CHIKV and ZIKV infections increased following their spread in the Americas. Notably, between 2016 and 2017, locally acquired CHIKV cases in Europe exceeded travel-related cases due to outbreaks in the Lazio and Calabria regions of Italy. The expansion of both infections and vectors increases the likelihood of mutual adaptation that further facilitates their spread. For instance, a new sublineage of CHIKV emerged from an A226V amino acid change in the ECSA E1 glycoprotein during the 2005–2006 Indian Ocean outbreaks (Indian Ocean Lineage: IOL), which was associated with enhanced viral multiplication of A. albopictus mosquitoes, facilitating their dissemination.1 Moreover, the movement or transport of viremic livestock and animals carrying infected ticks can introduce viruses like CCHFV into new geographic areas. Indeed, the spread of CCHFV from its African ancestor to other areas of the world appears to coincide in time with livestock movements. Changes in human practices, such as adapting water storage needs, in response to drought and handling inadequate domestic water supplies, can create new ecosystems for vector proliferation. Economic developments and global events, such as major epidemics, can also profoundly impact the distribution of arboviral disease risks and strain vector control and public health programs, as observed during the COVID-19 pandemic.1,2,12
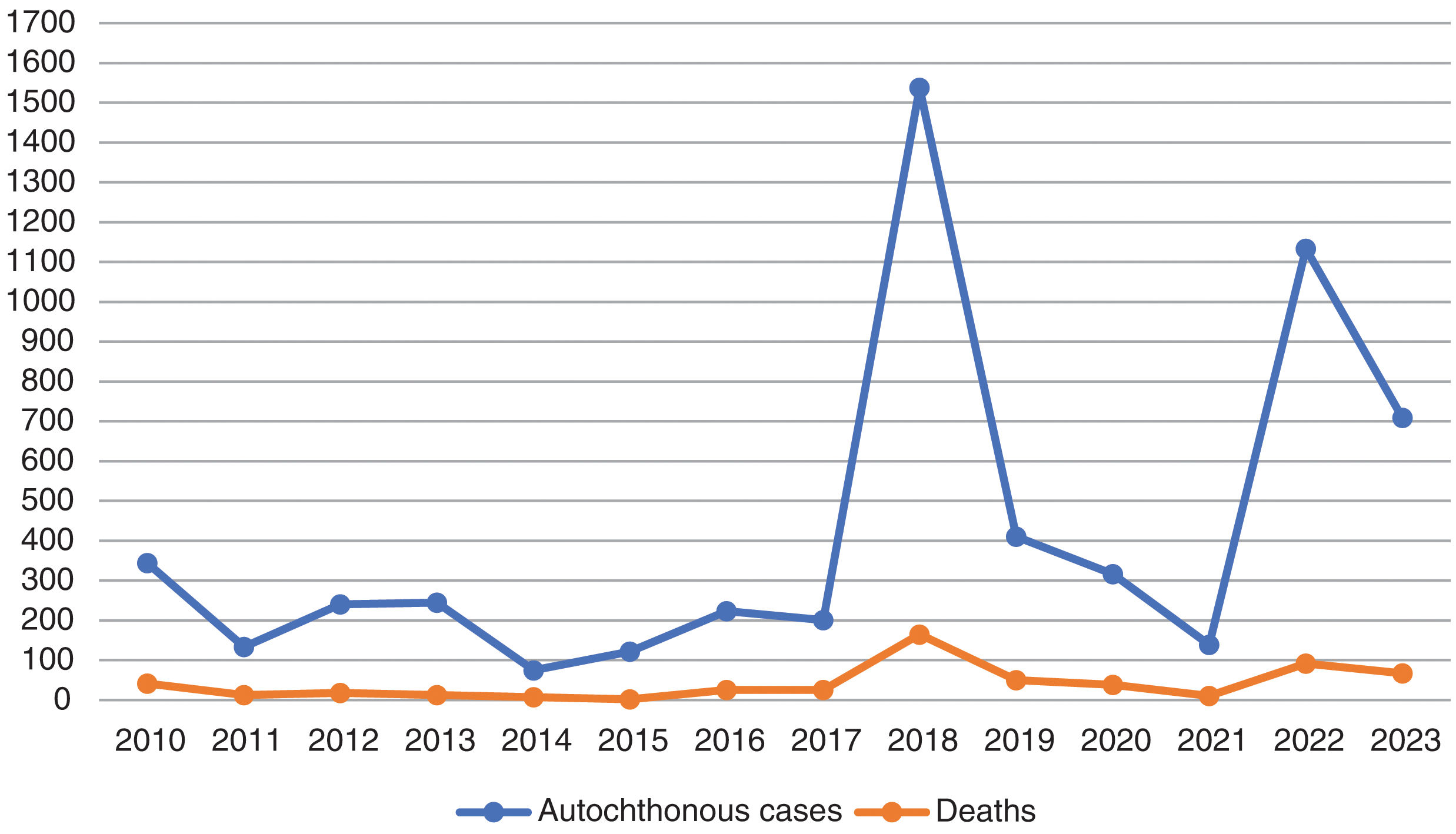
Individualized analysis of the risk of expansion of arboviruses in EuropeWNV has caused recurring annual outbreaks since the early 2000s, with hundreds of cases reported each year across Southern and Eastern Europe. In 2023, nine European Union countries reported a total of 709 locally acquired human cases of WNV infection, including 67 fatalities. Although the number of cases in 2023 is lower than in the previous year, the spread of the virus in 2023 has reached the greatest number of regions since the 2018 peak, which indicates a significant geographical expansion of the virus across Europe (Table 1 and Fig. 1).23,24
Cases of West Nile virus infection in the European Union (EU), including probable and confirmed autochthonous human cases, following the EU case definition (Commission Decision 2008/426/EC).23,24
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 1 | 2 | 6 | 5 | 6 | 19 | 4 | 3 | 6 | |||||
| Bulgaria | 2 | 1 | 1 | 15 | 5 | 1 | ||||||||
| Croatia | 6 | 20 | 1 | 1 | 2 | 5 | 57 | 8 | 6 | |||||
| Cyprus | 1 | 1 | 16 | 5 | ||||||||||
| Czech Republic | 3 | |||||||||||||
| France | 1 | 2 | 27 | 2 | 6 | 43 | ||||||||
| Germany | 4 | 13 | 4 | 16 | 6 | |||||||||
| Greece | 262 | 100 | 157 | 85 | 15 | 48 | 311 | 223 | 143 | 57 | 283 | 162 | ||
| Hungary | 18 | 4 | 17 | 35 | 10 | 18 | 42 | 20 | 213 | 36 | 3 | 7 | 14 | 29 |
| Italy | 4 | 18 | 45 | 80 | 24 | 61 | 76 | 53 | 610 | 53 | 66 | 55 | 723 | 336 |
| Netherlands | 7 | |||||||||||||
| Portugal | 1 | |||||||||||||
| Romania | 57 | 11 | 15 | 24 | 23 | 32 | 93 | 66 | 277 | 66 | 6 | 7 | 47 | 103 |
| Slovakia | 1 | 1 | ||||||||||||
| Slovenia | 1 | 4 | ||||||||||||
| Spain | 2 | 3 | 77 | 6 | 4 | 19 | ||||||||
| Total | 344 | 133 | 240 | 245 | 75 | 122 | 223 | 201 | 1537 | 410 | 316 | 139 | 1108 | 709 |
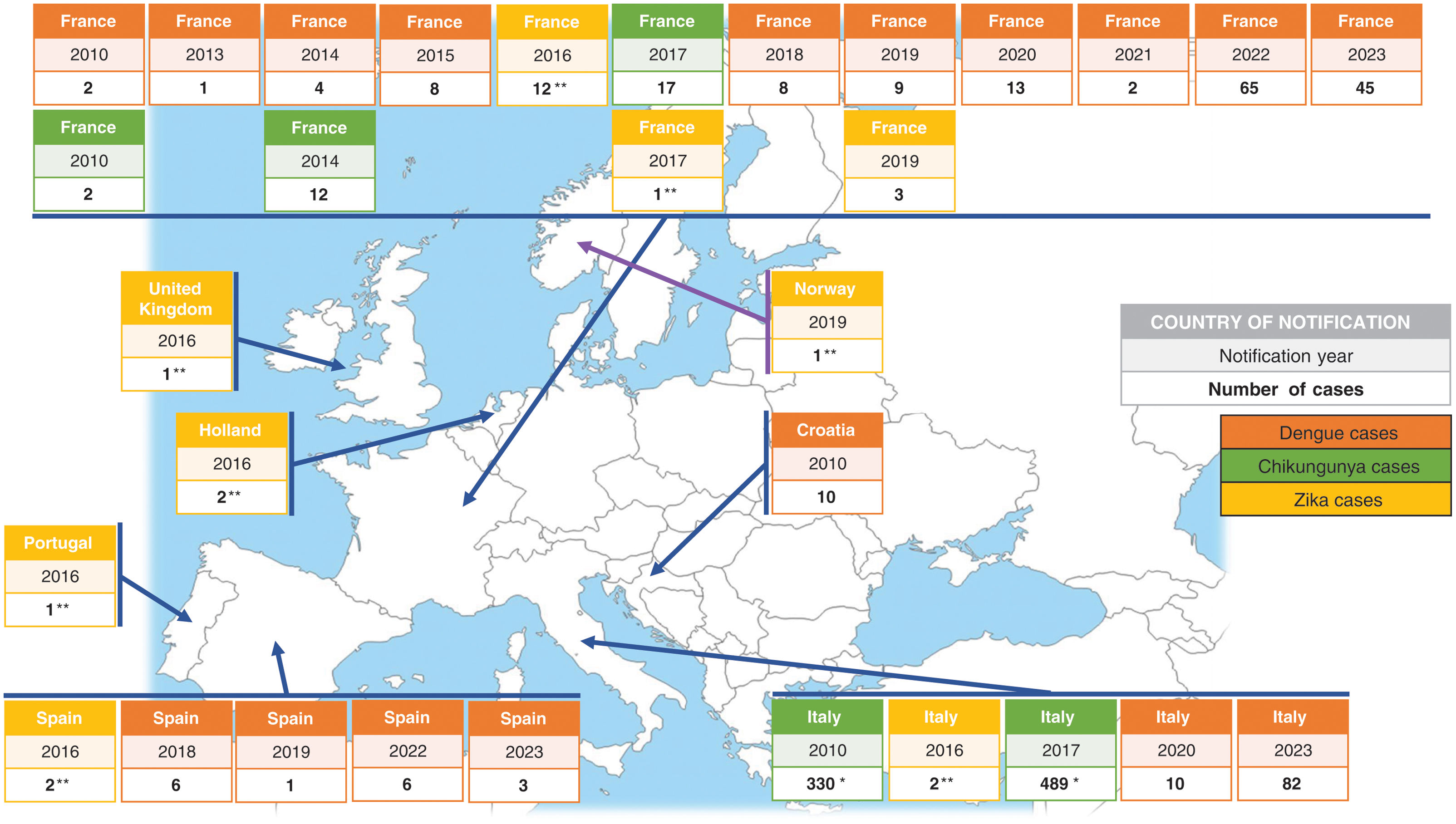
CHIKV and DENV cases have also been reported in Europe, although less frequently, with several outbreaks linked to local transmission from viremic travelers returning from endemic areas (Fig. 2). According to the World Health Organization, an estimated 390 million people worldwide are infected with DENV annually, of which 96 million show clinical symptoms. Over 125 countries, encompassing more than 50% of the global population, are at potential risk of infection. In 2023, the mainland EU/EEA reported 130 cases of locally acquired DENV, doubling the total number of cases reported from 2010 to 2021. Furthermore, although sporadic, autochthonous cases of ZIKV have been identified in France. The presence of other arbovirus, such as USUV and CCHFV, has also been established in Europe despite their origins in other continents (Table 2). The likelihood of secondary transmission of OROV within continental Europe is considered very low due to the absence of known competent vectors that are commonly found in the Americas. These instances highlight the growing risk of establishment of arboviruses in Europe, both by the expansion of competent mosquito vectors and by the increase in the global mobility.2,25–28
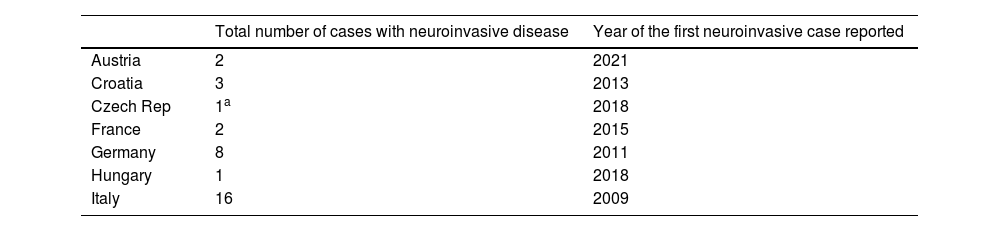
Cases of Usutu virus (USUV) infection with neuroinvasive disease in the European Union (EU).24,25
| Total number of cases with neuroinvasive disease | Year of the first neuroinvasive case reported | |
|---|---|---|
| Austria | 2 | 2021 |
| Croatia | 3 | 2013 |
| Czech Rep | 1a | 2018 |
| France | 2 | 2015 |
| Germany | 8 | 2011 |
| Hungary | 1 | 2018 |
| Italy | 16 | 2009 |
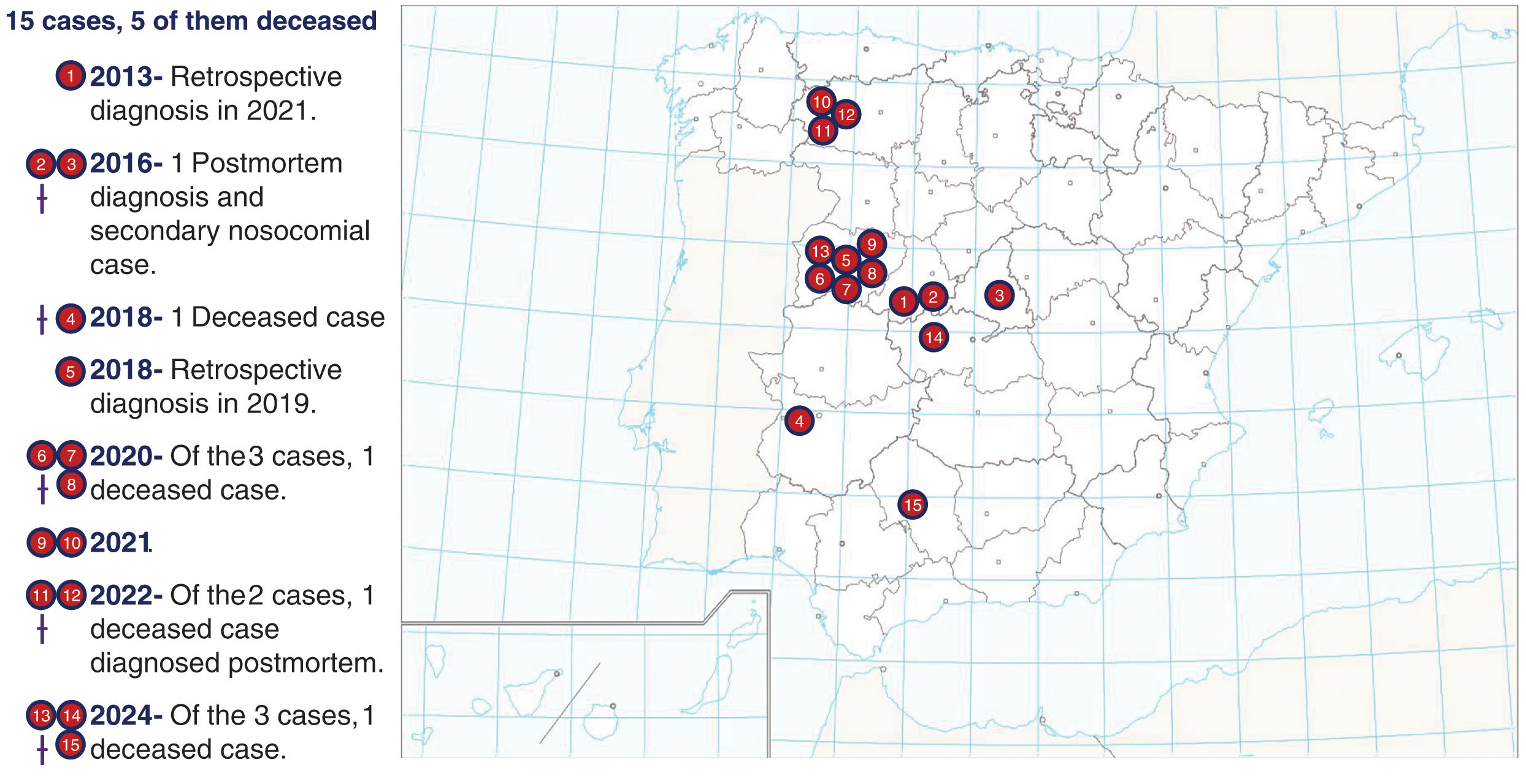
In Spain, the current arboviral landscape is characterized by low numbers of DENV, CCHFV (Fig. 3), and WNV cases. However, projections indicate an upward trend in risk due to factors such as climate change, increased movement of people, and the proliferation of vector habitats. Epidemiological data from 2017 to 2023 indicate 16 locally acquired cases of DENV, 37 cases of WNV and 14 cases of CCHFV. Although these number represent a minimal impact in terms of morbidity and mortality, proactive measures are needed to prevent an escalation.29,30 Key strategies include enhancing vector surveillance and control, strengthening public health education on preventive measures, and improving diagnostic capabilities. In addition, the identification of travelers arriving in areas where competent vectors for disease transmission exist, such as Europe/Spain, is critical in preventing the local establishment of the disease. The implementation of surveillance and monitoring at entry points, coupled with informed traveler screening procedures, can significantly reduce the risk of introducing and spreading arboviruses to new regions. Documenting travelers’ recent visits to endemic areas in their medical history is essential for promptly identifying potential carriers of arboviruses.31
New therapeutics and vaccines for arboviral diseasesIndividual and community measures remain the most important, therefore, they must be implemented to prevent and control these infections: protective measures at both individual and collective behavioral levels, the use of insecticides, public education, blood and blood products control, biological vector control, and the quarantine of infected individuals. Moreover, pre-travel training is essential: information regarding endemic and transmissible infections should be provided during a pre-travel consultation with health professionals, although data shows that many travelers do not attend a pre-travel consultation (for example, in one study the authors found that 50% of attendees at a traveler advice consultation would not have attended the visit if they had known that the 10-year booster for yellow fever was no longer necessary).1,2
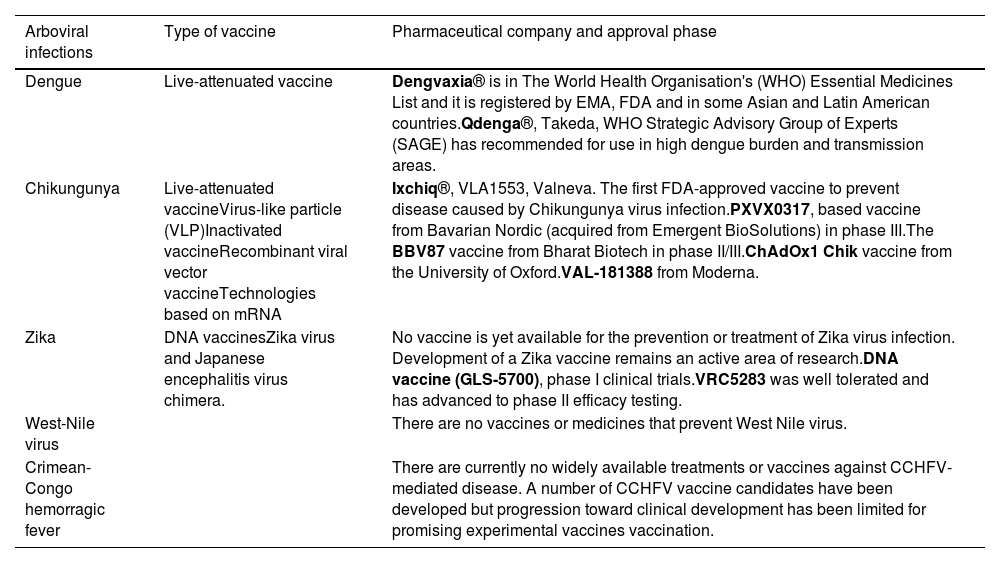
Currently, no specific antiviral treatment exists for DENV, ZIKV, CHIKV or WNV infection. Management primarily focuses on relieving symptoms a providing supportive care. In severe cases, hospitalization may be necessary to monitor complications as pleural or pericardial effusion or neurological symptoms as meningitis or encephalitis. Without an effective treatment for these arboviruses, it is a priority to continue research in this direction and develop vaccines, which produce stable and long-lasting protective antibodies against each serotype of the virus to prevent severe immune reactions due to infections caused by different serotypes (see summary in Table 3).
Summary of the present situation of the most relevant vaccines and treatments for arboviral infections.32–51
| Arboviral infections | Type of vaccine | Pharmaceutical company and approval phase |
|---|---|---|
| Dengue | Live-attenuated vaccine | Dengvaxia® is in The World Health Organisation's (WHO) Essential Medicines List and it is registered by EMA, FDA and in some Asian and Latin American countries.Qdenga®, Takeda, WHO Strategic Advisory Group of Experts (SAGE) has recommended for use in high dengue burden and transmission areas. |
| Chikungunya | Live-attenuated vaccineVirus-like particle (VLP)Inactivated vaccineRecombinant viral vector vaccineTechnologies based on mRNA | Ixchiq®, VLA1553, Valneva. The first FDA-approved vaccine to prevent disease caused by Chikungunya virus infection.PXVX0317, based vaccine from Bavarian Nordic (acquired from Emergent BioSolutions) in phase III.The BBV87 vaccine from Bharat Biotech in phase II/III.ChAdOx1 Chik vaccine from the University of Oxford.VAL-181388 from Moderna. |
| Zika | DNA vaccinesZika virus and Japanese encephalitis virus chimera. | No vaccine is yet available for the prevention or treatment of Zika virus infection. Development of a Zika vaccine remains an active area of research.DNA vaccine (GLS-5700), phase I clinical trials.VRC5283 was well tolerated and has advanced to phase II efficacy testing. |
| West-Nile virus | There are no vaccines or medicines that prevent West Nile virus. | |
| Crimean-Congo hemorragic fever | There are currently no widely available treatments or vaccines against CCHFV-mediated disease. A number of CCHFV vaccine candidates have been developed but progression toward clinical development has been limited for promising experimental vaccines vaccination. |
Recent advancements in the therapeutic landscape and vaccine development are vital in the ongoing battle against DENV. Over the past decade, there has been a significant push to identify antiviral compounds that can be advanced into clinical trials, with a focus on direct-acting antivirals and host-directed therapies. While several promising candidates have been tested, including chloroquine, celgosivir, lovastatin, balapiravir, or prednisolone, the development of an effective treatment regimen has been prevented by the need for efficacy against all serotypes and the complex pathogenesis of the disease. In that sense, the novel molecule JNJ-1802, a pan-serotype DENV antiviral small molecule, has shown promising results.32–34 The search for a DENV vaccine has yielded more promising results. The first licensed vaccine, Dengvaxia®, marked a milestone in DENV infection prevention, yet its application was limited by age and serostatus of recipients. The newly authorized Qdenga® is suitable for individuals aged 4 and above (including travelers to endemic areas), regardless of baseline DENV immune status, although there are concerns regarding its safety and efficacy against certain serotypes. Current candidates in clinical trials, such as the phase-III Butantan-DV vaccine, show promise with their live-attenuated formulations. Beyond these, over a dozen vaccine candidates employing various immunization strategies are undergoing pre-clinical trials, enriching the pipeline with innovative approaches to traditional DENV vaccines. The challenges to develop a pan-serotype vaccine are compounded by the potential for antibody-mediated disease enhancement, which has been observed in clinical settings. Understanding this immune response to DENV is essential for the development of next-generation vaccines that can provide safe and effective immunity.35–37
ChikungunyaThere is no specific antiviral available to control CHIKV replication, therefore, advanced research is oriented toward prevention. A major problem is that not all serotypes of the CHIKV are known, which could lead to less effective CHIKV vaccines. Currently, in Europe, there are no authorized vaccines for CHIKV infection, although several promising candidates are under development.1 In the USA, a live attenuated vaccine (Ixchiq®, VLA1553, Valneva) consisting of a single intramuscular dose that induces a seroresponse of 97.8% at 28 days post-vaccination has recently been approved. Although no efficacy data are yet available, the rates of local and systemic adverse events are similar to these of many other vaccines already in use. The vaccine is not yet marketed in Europe, but the European Medicines Agency has granted an accelerated assessment. This vaccine would be recommended for travelers aged ≥18 years arriving in areas with an active CHIKV outbreak and may be considered for those over 65 years of age who will have at least moderate exposure (at least 2 weeks) to mosquitoes, or persons staying for a cumulative period of 6 months or more in an endemic area, even though there may not be an active outbreak. Other promising candidates include PXVX0317 from Bavarian Nordic in phase III, which is a virus-like particle (VLP) based vaccine, administered to a population aged between 12 and 64 years in a single intramuscular dose. The BBV87 vaccine from Bharat Biotech, currently in phase II/III, is an inactivated vaccine containing the CHIKV strain derived from the ECSA genotype, administered in two intramuscular doses 28 days apart, and is being tested in Panama, Colombia, Thailand, and India. The MV-CHIK-202 vaccine from Themis/Merck, currently in phase II (although recently discontinued) is a recombinant live attenuated vaccine containing the measles virus vaccine strain, and has been tested in Germany, Austria and Puerto Rico in volunteers over 18 years old, in one or two intramuscular doses 28 days apart. Other vaccines in earlier phases of research include the ChAdOx1 Chik vaccine (a recombinant viral vector vaccine using a chimpanzee adenovirus) from the University of Oxford, and theVAL-181388 from Moderna Technologies based on mRNA.38–40
Many compounds have shown efficacy against CHIKV in vitro, but to date, there are no specific licensed therapeutics against CHIKV. The current therapeutic objectives for patients with CHIKV infection and its chronic symptoms are to relieve pain, reduce inflammation, and limit joint stiffness with the use of analgesics, anti-inflammatory drugs (at least two weeks and up to 1 month after onset) and maintain hydration and general rest. Physiotherapy is often necessary to restore lost muscle tone and physical fitness due to the illness. Several clinical guidelines have been developed for the treatment of acute or chronic symptoms of chikungunya and recommend the use of common analgesics or weak opioids during the acute phase, and non-steroidal anti-inflammatory drugs (NSAIDs) with or without adjuvant drugs during the subacute phase. During the chronic phase, analgesics, weak opioids, NSAIDS and low dose oral steroids are recommended. Disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, with or without sulfasalazine or hydroxychloroquine, in addition to steroids, are recommended in patients with chronic symptoms with inflammatory disease. Biologics can be used in patients who are refractory to steroids and DMARDs.1
ZikaImmunopathological events significantly influence vaccine development for ZIKV, notably when DENV co-circulates, enhancing ZIKV replication via Fcg receptor-mediated entry. This interaction, facilitated by DENV-induced anti-FLE antibodies, exacerbates ZIKV symptoms. Vaccine strategies must focus on protecting vulnerable groups like pregnant women while avoiding fetal harm.38
There are various vaccines in development based on different biological strategies. One of the most promising inactivated ZIKV vaccines has achieved high antibody titers in its participants, although these titers decreased from day 43 to day 57. Regarding DNA-based vaccines, a new DNA vaccine (GLS-5700) was tested in 40 participants, and two weeks after the third dose, all participants produced specific antibodies for ZIKV. However, in 60% of the participants, their neutralizing titers were not high enough. Another DNA vaccine in development is VRC5283 (chimeric with Japanese encephalitis virus) and VRC5288. The results revealed that both were safe and well tolerated, and neutralizing titers were observed in 100% of participants. Among the mRNA vaccine techniques, ZIKV Brazil (mRNA-LNP) currently stands out. mRNA vaccines avoid the risks of infection and insertional mutagenesis, as well as anti-vector immunity. Moreover, mRNA vaccines are more efficiently expressed by their target cells. In murine models, they induce protective antibodies in immunized animals without causing post-vaccination side effects. In rhesus macaques, they generate potent neutralizing antibodies and specific antibodies against ZIKV, which provide complete protection. A series of vaccines based on viral vectors, such as those using an attenuated measles virus vector or chimpanzee adenovirus AdC7, caused injected mice to produce immunoglobulins against ZIKV. Finally, among the strategies to develop a live attenuated vaccine, two mechanisms are used: the mutation of a real ZIKV isolate to produce attenuating mutations and to create chimeric flaviviruses using Japanese encephalitis virus or yellow fever virus. In non-human animal testing phases, these vaccines seem to achieve neutralizing antibody titers and sterilizing immunity in monkeys, showing good safety profiles.38,41
OropoucheCurrently, there is no specific antiviral treatment or vaccine available for OROV. Ribavirin and mycophenolic acid are ineffective in vivo, and while interferon-alpha can limit viral replication in mice, its clinical relevance is unclear. A promising vaccine candidate using a chimeric vesicular stomatitis virus expressing OROV glycoproteins has shown potential in mice, and computational methods have identified novel epitopes for vaccine design. Cross-protection within the Simbu serogroup remains limited, as vaccines for related viruses like Schmallenberg (licensed for veterinary use) have shown mixed efficacy.3
West-NileOngoing research is exploring potential therapeutic avenues. Additionally, monoclonal antibodies targeting specific viral proteins are being investigated for their ability to neutralize WNV and reduce disease severity. Recombinant human GM-CSF (rHuGMCSF) is an FDA-approved drug used to increase the number of white blood cells and has been used in mice models to prevent neurological complications. Despite the common use of steroids in cases of encephalitis, studies showed no benefits. In this context, intravenous immunoglobulins can be effective to reduce morbidity and mortality in neuroinvasive WNV infection.42–44
The most promising approach for preventing WNV infection lies in vaccination. One vaccine based on an inactivated form of the virus, or another based on a subunit vaccine, have shown encouraging results in clinical trials. These vaccines have demonstrated safety and immunogenicity in human volunteers, eliciting a robust immune response against WNV. Further research is needed to optimize vaccine formulations and determine long-term efficacy.45
Crimean-Congo hemorragic feverSome antiviral drugs have shown promise against Crimean-Congo hemorrhagic fever (CCHF). Ribavirin, a nucleoside analog, has long been used as a direct-acting antiviral for treating CCHF, but its efficacy remains debated due to confounding factors in clinical data and mixed results in animal studies. Systematic meta-analyses suggest its benefits are questionable and likely dependent on early treatment after exposure, which is challenging outside of controlled settings. Favipiravir has shown efficacy in animal models, but limited data are in humans. The recently reported T-705-derived compound, H44 can inhibit CCHFV infection in vivo and in vitro. No clinical data are available for monoclonal antibodies. Other potential treatments like 2′-deoxy-2′-fluorocytidine and molnupiravir show varying degrees of preclinical efficacy, with the latter not protecting against infection in animal models. Antibody-based therapies for CCHFV show promise, particularly from survivor plasma and monoclonal antibodies, although results vary across models. While some antibodies protect neonatal mice, their effectiveness in adult models and post-exposure scenarios is limited, highlighting the need for further research to optimize therapeutic approaches.46–48 Studies indicate that anti-inflammatory treatments, such as high-dose methylprednisolone with ribavirin, may improve outcomes by modulating this response. Limited cohort sizes and promising animal model results suggest further investigation is needed for therapies of TNF blockers and other cytokine-targeting therapies.49,50
The development of a safe and effective vaccine against CCHFV is a top priority for the global health authorities. One promising candidate is a live attenuated vaccine derived from an avirulent strain of CCHFV. This vaccine has demonstrated efficacy in animal models eliciting strong immune responses and conferring protection against the lethal challenge with virulent CCHFV. Clinical trials in Turkey are underway to assess its safety and immunogenicity in humans.51
Challenges and advances in diagnosing arbovirus infectionsThe laboratory diagnosis of arboviruses depends on understanding the dynamics of each arboviral infection. Combining serological tests with direct virological techniques enhances the likelihood of diagnosing arboviral diseases. However, to interpret results accurately, it is crucial to have reliable data regarding the timing of onset of symptoms, sample collection, and vaccination history against flaviviruses.
Molecular-based detectionReverse transcription polymerase chain reaction (RT-PCR) has been widely used in diagnosing arboviral diseases during the febrile stage due to its high sensitivity and specificity. Nevertheless, its widespread use at point-of-care (POC) settings is limited by the need for specialized equipment, infrastructure, and trained personnel. POC systems are crucial both in resource-limited and rich settings to avoid empirical treatments and to prevent potential complications. Furthermore, their ability to provide rapid diagnosis facilitates the implementation of control strategies and supports public health surveillance. Examples of POC systems based on RT-PCR that use a syndromic approach for the diagnosis of acute febrile illnesses in tropical and subtropical regions include VereFever™ (Veredus Laboratories, Singapore. Research use only), which can detect the presence of CHIKV, DENV 1–4, JEV, WNV, YFV, ZIKV, and various species of Plasmodium on a single chip, and the BioFire® Global Fever Panel (BioFire, Salt Lake City, USA), whose recent evaluations have shown excellent performance for several analytes, including Plasmodium spp., DENV, and CHIKV, although the prototype panel failed to detect ZIKV infections. Despite their potential, these systems are expensive (>100€/sample), which complicates their widespread use.52,53 Another POC panel intended for diagnosing arbovirus infections is the STANDARD™ M10 Arbovirus Panel (SD Biosensor Inc., Suwon, Korea), a multiplex real-time RT-PCR that enables detection of DENV (differentiation of 4 serotypes), ZIKV, CHIKV, YFV and WNV. However, no evaluations of this panel have been published yet. It is very important to highlight that the sensitivity of RT-PCR for CCHFV is influenced by its genetic diversity, which correlates with different geographical areas. This implies that some assays may be suitable for diagnosis of CCHF in the regions where circulating strains were studied, whereas infections due to other clades may be missed.46,54
Isothermal nucleic acid amplification technologies (INAATs) are an affordable approach that can amplify nucleic acids at a fixed temperature, eliminating the need for thermal cycling equipment. These methods hold the potential to decentralize molecular diagnostic testing, facilitating rapid and cost-effective testing suitable for resource-limited settings. Among these techniques, loop-mediated isothermal amplification (LAMP) is the most utilized method. Several LAMP techniques have been developed for detecting arboviruses, positioning LAMP technology as an alternative to RT-PCR for diagnosing these viral infections.55–57
Metagenomic next generation sequencing (mNGS) is increasingly being employed as a diagnostic tool, especially in patients presenting with undiagnosed encephalitis/meningitis and in returning travelers with undiagnosed acute undifferentiated febrile illnesses. This technique is also being used for surveillance and has become a mainstay in characterizing and profiling mosquito viromes and investigating virus diversity in ticks. However, this approach is laborious, expensive and needs an specialized equipment. Moreover, interpretation of mNGS results is challenging and requires expertise.58–60
Serology-based diagnosisBeyond the febrile stage, direct detection methods of arboviruses are ineffective. As a result, serology becomes essential for diagnosing these infections. The presence of cross-reactive antibodies in flavivirus infections can lead to false positive results. A recent review revealed that cross-reactivity with DENV was observed in approximately 15.4–84% of antibodies produced against non-DENV flaviviruses across various assays. In addition, up to 30% of IgM and up to 60% of IgG antibodies generated against non-WNV flaviviruses showed cross-reactivity with WNV on enzimo-immunoanalysis assays (EIA). Conversely, the level of antibodies produced against flaviviruses that cross-react with CHIKV (an Alphavirus) is notably low, approximately 7%. The highest antibody cross-reactivity of flaviviruses was reported in IgG-based assays compared to IgM-based assays and assays based on E-specific immunoglobulin compared to NS1-specific immunoglobulin.61
The gold standard confirmatory assay is the plaque reduction neutralization test (PRNT). This method quantifies the degree to which cellular infection is inhibited by neutralizing antibodies present in serum or in cerebrospinal fluid. Results provide a direct estimate of the quantity of functional antibodies, and are valuable not only for confirming recent infection, but also for vaccine development. However, the serological diagnosis of arboviruses is challenged by previous infections. In secondary or subsequent infections with similar flaviviruses, the phenomenon known as “original antigenic sin” alters immune responses, resulting in a lower avidity for antigens of the infecting virus. For instance, infection with DENV or ZIKV in patients who have been infected with DENV before, IgM titers are notably reduced, heightening the risk of false-negative test results. Moreover, in secondary DENV infections, the sensitivity of antigen-detecting tests is reduced by roughly 30%, probably because of antigen depletion by pre-existing antibodies.62 It is important to note that in severe CCHF cases, antibody production can be delayed or even absent, potentially escaping detection through ELISA or immunofluorescence assays. Incorporating both direct viral testing and serological testing during the second week of illness and thereafter would reduce the probability of overlooking a CCHF diagnosis.46,54 These findings highlight the increasing challenge in interpreting serology-based diagnostics, support the need to conduct epidemiological investigations due to the global spread of arboviruses, and bring forth the urgent need for the development of more specific serological techniques.63,64
ConclusionsPublic Health authorities play a crucial role in minimizing arbovirus infection risks in vulnerable areas by identifying environmental risk factors and assessing how climate change influences these infections. It is essential to use a multispecies approach to characterize transmission scenarios and understand virus reservoirs, particularly in temperate climates where the virus can be maintained during mosquito hibernation. In the absence of specific antivirals, developing and monitoring vaccines is essential for managing arbovirus spread. These efforts highlight the evolving nature of arbovirus diagnosis and the need for continuous innovation to meet emerging challenges and ensure accurate, timely diagnoses across various contexts. As an example of the dynamism and the continuous need to be updated on the global perspective of arboviruses, we have observed the current outbreaks of the OROV in Latin America and the first imported cases diagnosed in Europe: in July 2024, three confirmed cases of OROV infection were reported in three different regions of Spain in travelers arriving from Cuba.65
FundingAll authors certify that we have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Conflicts of interestAll authors: No reported conflicts of interest.