Multidrug resistance among bacteria increases the need for new therapeutic options. Tigecycline is one candidate drug, due to property of a wider anti-bacterial spectrum to multi-drug resistant (MDR) pathogens. However, it has still not been approved for use in pediatric patients.
MethodsIn this study the effectiveness and safety of tigecycline in children was assessed retrospectively.
ResultsA total of 36 pediatric patients, received tigecycline therapy with a median of 13 days (2–32 days). Tigecycline was used as a combination therapy in all cases. Microbiological eradication was achieved in 27 patients (75%) and clinical response was observed in 30 patients (83%). There were six cases (17%) of relapse.
ConclusionOur findings suggest that tigecycline may be an option for children with severe infections due to multidrug resistant bacteria.
La multirresistencia por parte de las bacterias aumenta la necesidad de nuevas opciones de tratamiento. La tigeciclina es un fármaco candidato, debido a la propiedad de presentar un espectro antibacteriano más amplio frente a patógenos multirresistentes. Sin embargo, todavía no se ha aprobado para su uso en pacientes pediátricos.
MétodosEn este estudio se evaluó de forma retrospectiva la eficacia y la seguridad de la tigeciclina en niños.
ResultadosUn total de 36 pacientes pediátricos recibieron tratamiento con tigeciclina durante una mediana de 13 días (2-32 días). La tigeciclina se utilizó como parte de un tratamiento combinado en todos los casos. Se consiguió la erradicación microbiológica en 27 pacientes (75%) y se observó respuesta clínica en 30 pacientes (83%). Hubo 6 casos (17%) de recidiva.
ConclusiónNuestros hallazgos sugieren que la tigeciclina puede ser una opción para niños con infecciones graves debidas a bacterias multirresistentes.
Infections with multi drug resistant (MDR) pathogens are associated with increased mortality, morbidity, and length and cost of hospital stay. Antimicrobial resistance has been progressive especially in Gram-negative pathogens. Therefore, any new antibiotics effective against strains resistant to existing drugs would gain worldwide attention. Tigecycline is one such antibiotic, with efficacy against many multidrug-resistant pathogens widely reported. The US Food and Drug Administration (FDA) has approved tigecycline use for complicated intra-abdominal infections and complicated skin and skin structure infection (2005), and for community-acquired pneumonia (2009).1,2 Uses besides these indications and in pediatric patients has not currently been approved. However, when no alternative antibacterial drugs are available for severe infections, tigecycline may be used with due consideration to the risk–benefit ratio.
In this study, we retrospectively analyzed the children with life-threatening infections caused by MDR pathogens, receiving tigecycline therapy in a tertiary children's hospital in Turkey.
Material and methodsPatients aged ≤18 years who received ≥2 days of tigecycline therapy between March 2015–March 2018 were included in the study. Patients who received intravenous tigecycline therapy for culture documented infection were included in the study and were evaluated retrospectively (Except 2 patients who had sepsis with unknown etiology ınresponsive to any other antibiotics). The patients who received <4 doses of intravenous tigecycline were excluded.
Multidrug resistance was defined as resistance to at least three antimicrobial drugs of different antimicrobial categories. There are no CLSI MIC breakpoints for tigecycline to Acinetobacter spp. So the same FDA breakpoints that were set for Enterobacteriaceae were used for A. baumannii as well.
Data collected for each patient included: age; sex; diagnosis; side effect if present; use of concomitant antibiotics if present, adverse events if present, outcome; and mortality. Tigecycline was manufactured and distributed by Pfizer®. Tigecycline was given with a dose of 1mg/kg/day in 2 doses with the approval of off-label use by Turkish Medicines and Medical Devices Agency. The study was approved by Local Ethics Committee.
All statistical analysis was performed with SPSS version 17.0 (SPSS Inc, Chicago, IL, USA). Quantitative variables were expressed as the mean±standard deviation or median(range) and qualitative variables were expressed as number and percentage. The Mann–Whitney U-test was used for quantitative variables, and differences between qualitative variables were analyzed by analyzed by chi square test. A p value <0.05 was considered to be statistically significant.
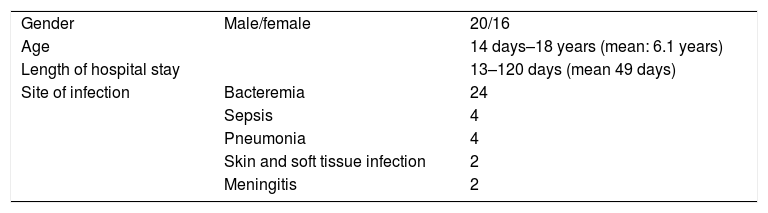
ResultsA total of 36 pediatric patients, 16 female (44%), 20 male aged between 14 days–18 years (7 patients were in newborn period) (median: 5.5, mean: 6.1 years) were administered tigecycline therapy (Table 1).
Diagnosis of the patients requiring tigecycline administration were reviewed in Table 1. Patients received tigecycline therapy with a median of 13 days (2–32 days) (mean: 14.53±7.6 days). All of the microorganisms were resistant to carbapenems. No adverse effect, due to tigecycline therapy was observed. One neonate and 1 burn patient received tigecycline although no microorganism was detected as they were septic and were hospitalised close to a multidrug resistant A. baumanii. After initiation of tigecycline therapy both of the patients were cured clinically, so it was considered that etiology could be due to multidrug resistant microorganism which could not be cultivated due to previous antibiotic usage.
Accompanying antimicrobial distribution was as follows: amikacin in 1 patient, meropenem was used in 13 patients, imipenem in 5 patients, piperacilin-tazobactam in 4 patients, colistin in 24 patients, ciprofloxacin in 4 patients, rifampicin in 3 patients, intraventricular colistin in 2 patients.
Microbiological eradication was achieved in 27 patients (75%) and clinical response was observed in 30 patients (83%). Therapy failed in 6 patients (17%) which ended with relapse. Mortality rate of the patients was 36% (13 patients). Seven patients died because of breakthrough infections due to tigecycline resistant bacteria (6 Pseudomonas spp and 1 Acinetobacter spp) while receiving tigecycline. These 7 patients were on combination therapy including colistin. Mortality in the rest 6 patients, could not have been attributed to failure of tigecycline. Outcome and mortality did not significantly differ according to diagnosis. Effectiveness of therapy and mortality was not significantly different according to microorganism. In subgroup analysis, examining neonates and burn patients effectiveness of therapy and mortality did not differ according to microorganism. Age did not have a significant effect on outcome and mortality (although number of patients at each section were few to make a clear statement).
DiscussionVery few clinical studies have specifically evaluated the use of tigecycline in pediatric patients, especially neonates. Our study is one of the largest series with 36 pediatric cases of which 7 were neonates and 19 were burn patients. In a literature review it was stated that tigecycline has been administered to 62 children with challenging infections caused by MDR strains and it showed a favorable clinical response rate of 74.2%, but except case reports, no data about infants and children aged less than 8 years old are available in literature.3
In a current study 37 pediatric patients with hematologic malignancies receiving tigecycline were evaluated, and improvement was observed in 48.7% of cases at the end of tigecycline therapy.4 Similarly, Ye et al. reported clinical improvement rate of 47.27% in preliminary experience of tigecycline therapy in 110 pediatric patients.5 In a study from China, cure rate of tigecycline was reported as 45.8%, and 29.2% of patients were switched to other antibacterial agents due to clinical unresponsiveness.6 In our study microbiological eradication was achieved in 27 patients (75%) and clinical response was observed in 30 patients (83%). Higher cure rates might have depended on the difference of underlying diseases of the patients in other series.
Although, tigecycline has the widest range of antibacterial activity, it is not effective for Pseudomonas aeruginosa. So during tigecycline therapy emerging resistant pseudomonal infection could complicate the therapy. In our study, 6 patients after initiation of tigecycline, resistant pseudomonas infection occured resulting with death of these patients.
A few reports have documented the pharmacokinetics of tigecycline in the literature. In animal studies tigecycline tissue levels, with the highest concentrations in bone, liver, spleen, and kidney, exceeded those in plasma and persisted longer.7 Also high dose tigecycline (100mg every 12h for adult patients) in critically ill patients with severe infections were evaluated and in the ventilator associated pneumonia subgroup the high-dose regimen was associated with better outcomes than conventional administration due to Gram-negative MDR bacteria while tigecycline was well tolerated.8
Combination of tigecycline with other antibacterials has been investigated with other antibacterials against a wide range of susceptible and multiresistant Gram-positive and Gram-negative bacteria. Consistent beneficial activity of tigecycline in combination with other antibacterials against multiresistant organisms, including vancomycin against penicillin-resistant S. pneumoniae in experimental meningitis, gentamicin against Pseudomonas aeruginosa in experimental pneumonia, daptomycin against Enterococcus faecium endocarditis, and colistin against K. pneumoniae bacteraemia and P. aeruginosa osteomyelitis have been documented from animal experiments and case reports. Antagonism was extremely rare in vitro and was not reported in vivo.9 In our study tigecycline was also used in combination therapy.
Furthermore, tigecycline targets two major resistance mechanisms—ribosomal protection and efflux pump mechanisms10,11 which will possibly making it the only choice for cases in which other antibiotic treatments have all failed.
The most important risk of tigecycline use might be increased mortality. The US Food and Drug Administration published drug safety guidelines in September, 201012 tigecycline is not approved for the use in children, it should not be used in pediatric patients unless no alternative antibacterial drugs are available. The European Medicines Agency (EMA) has approved tigecycline treatment in children above 8 years of age after consulting a physician with appropriate experience in the management of infectious diseases.3
Limitations of our study were; first as it was a retrospective study, effectiveness of tigecycline could have not be fully evaluated, second standard dose of tigecycline was used so, efficacy of higher doses can better be evaluated in future randomized controlled studies.
Our findings suggest that tigecycline may be an option for children with severe infections. However, more prospective, controlled trials are required to objectively evaluate the efficacy and safety of tigecycline in children.
Conflict of interestsNone declared.