Patients lacking humoral response have been suggested to develop a less severe COVID-19, but there are some reports with a prolonged, relapsing or deadly course. From April 2020, there is growing evidence on the benefits of COVID-19 convalescent plasma (CCP) for patients with humoral immunodeficiency. Most of them had a congenital primary immunodeficiency or were on treatment with anti CD20 antibodies. We report on three patients treated in our hospital and review thirty-one more cases described in the literature. All patients but three resolved clinical picture with CCP. A dose from 200 to 800ml was enough in most cases. Antibody levels after transfusion were negative or low, suggesting consumption of them in SARS-CoV-2 neutralization. These patients have a protracted clinical course shortened after CCP. CCP could be helpful for patients with humoral immunodeficiency. It avoid relapses and chronification. CCP should be transfused as early as possible in patients with COVID-19 and humoral immunodeficiency.
Se ha sugerido que los pacientes carentes de respuesta inmune humoral desarrollan una forma menos severa de COVID-19, pero existen algunos casos de curso prolongado, recurrente o incluso mortal. Desde abril de 2020 existen evidencias de los beneficios del plasma de convalecientes de COVID-19 (PCC) en los pacientes con inmunodeficiencia humoral. La mayoría tienen una inmunodeficiencia congénita primaria o están recibiendo tratamiento con anticuerpos anti-CD20. Describimos tres pacientes con inmunodeficiencia humoral y COVID-19 tratados con PCC en nuestro centro y revisamos los 31 casos más descritos en la literatura. Todos resolvieron el cuadro clínico con PCC, salvo tres. Una dosis de 200-800 mL fue suficiente en la mayoría de los casos. Los niveles de anticuerpos tras la transfusión fueron negativos o bajos, sugiriendo el consumo de los mismos en la neutralización del SARS-CoV-2. Estos pacientes tienen un curso clínico prolongado que se acorta tras la administración del PCC. El PCC podría ser de utilidad en los pacientes con inmunodeficiencia humoral. Evita las recaídas y la cronificación de la COVID-19. El PCC debería transfundirse lo antes posible en los pacientes con COVID-19 e inmunodeficiencia humoral.
Patients lacking humoral immune response must control infections relying on innate and cellular specific immunity. Monthly non-specific intravenous immunoglobulins (IVIg) can transfer immunity to these patients against most common infectious agents. However, being COVID-19 a new disease, immunity cannot be expected from non-specific IVIg. From March 2020, case series and systematic reviews described patients with COVID-19 treated with COVID-19 convalescent plasma (CCP). This therapy was safe and reduced mortality in critically ill patients, improved clinical symptoms and laboratory parameters, increased neutralizing antibody titers and negativized SARS-CoV-2-RNA.1 At this point, we considered that CCP could be crucial for the control of COVID-19 in those patients with humoral immunodeficiency (HI) at baseline.
Our objective was to analyze clinical, analytical, serological, virological and radiological evolution of patients admitted with COVID-19 suffering of an underlying HI treated with CCP; and review reports cited in the literature in this same setting.
MethodsFrom the beginning of the COVID-19 pandemic, plasma from convalescent patients was obtained by the “Red Andaluza de Medicina Transfusional, Tejidos y Células”, belonging to the “Sistema Sanitario Público de Andalucía” to be used in COVID-19 patients. This specific study was reviewed and approved by the “Comité de Ética de la Investigación de los Hospitales Virgen Macarena y Virgen del Rocío de Sevilla, Spain” (C.P.PH-SCoV-2-RAMTTC-C.I.1317-N-20). From May onwards, CCP was at disposal for randomized use in COVID-19 patients (clinical trial) and for patients with specific clinical conditions (observational study). Since then, during first COVID-19 wave, every patient with COVID-19 and HI was offered CPP as treatment for the disease. Patients gave written consent. HI was defined as the inability of the immune system to elaborate an antibody response, and could be primary/congenital or secondary, including B-cell depleting therapy. Every CCP unit had 300ml and was administered during 3–4h with no premedication. A second 300ml dose was considered 4–6 days after first one, if patients had no serum antibodies after first transfusion. This plasma was obtained following specific recommendations of the Spanish Ministry of Health: (1) Donors had recovered from COVID-19 and had a negative SARS-CoV-2 RNA in a nasopharyngeal swab 14 days before donation. (2) As a preventive measure for avoiding transfusion related acute lung injury (TRALI), donors with previous transfusions were rejected. Women were refused if previous pregnancies or abortions unless antibodies against HLA/HPA/HNA negative. (3) Plasma was matched by ABO group. (4) Transmissible infectious diseases were discarded. (5) Plasma was obtained by plasmapheresis. (6) Presence of antibodies IgG against SARS-CoV-2 was confirmed in plasma determining antibodies by ELISA.2
Plasmatic IgG against Spike glycoprotein and nucleocapsid protein of SARS-CoV-2 in CCP was determined following the insert of COVID-19 ELISA IgG, Vircell, Granada, Spain in the Microbiology Laboratory of the Hospital Universitario Clínico San Cecilio in Granada. Briefly, CCP optical density (OD) is determined simultaneously to a positive (OD>0.9), a negative (OD<0.5), and two cut-off controls (OD>0.55, OD<1.5). As OD saturates over 3, and cut-off media is usually around 0.6, OD CCP/cut-off index has a maximal value around 5. As a modification to Vircell insert, index was not multiplied by 10 on these samples. For a better titration of antibody levels, previously 47 samples of CCP were diluted 1/250, 1/500 and 1/1000, determining OD again. Two out of six samples with indexes ranging from 0.874 to 1.945 had OD positive, at a dilution 1/250 (1.222 index) and 1/1000 (1.945 index). All the thirteen samples with indexes ranging from 2 to 3 had OD positive, four at a dilution of 1/250, three at a dilution of 1/500 and six at a dilution of 1/1000. All the twelve samples with indexes ranging from 3 to 4 had OD positive, three at a dilution of 1/500 and nine at a dilution of 1/1000. All the sixteen samples with indexes ranging from 4 to 5.237 had OD positive, one at a dilution of 1/500 and fifteen at a dilution of 1/1000 (laboratory data, not published). Indexes over 1.5 defined CCP as hyperimmune and correlates with anti-IgG titers above 1/250 in 91.4% of samples, 1/500 in 80.8% of samples, and 1/1000 in 65.9% of samples. Recently it has been established a correlation between OD positivity at different ELISA titers and neutralizing activity of serum samples. Then, an ELISA titer over 1/320 correlates with neutralizing activity in 90% of sera.3 So, CCP with an index over 1.5 could have neutralizing activity in more than 80.8% of donations. In fact, as most donations have an index really higher, neutralizing activity is warranted.
Clinical evolution was evaluated daily and analytical, virological and radiological evolution at physician's discretion. Serological evaluation of patients included COVID-19 antibody determination by IgG/IgM immunochromatographic assay (ICA) (Healgen Scientific, Houston, Texas, USA; or Lambra, Zhejiang Orient Gene Biotech, China) and by IgG/IgM+IgA ELISA (Vircell, Granada, Spain). Following the insert recommendations with ELISA, the antibody index (OD plasma of the patient/media of OD cut-off control) was multiplied by 10. Vircell insert considers IgG indexes under 4 negative, 4-6 doubtful, and over 6 positive; IgM+IgA indexes under 6 negative, 6–8 doubtful, and over 8 positive for inactivated samples, as ours. SARS-CoV-2 was investigated in nasopharynx or sputum with VIASURE RT-PCR or with cobas® RT-PCR 6800.
ResultsDuring first COVID-19 wave, from early March to mid-June, 477 patients were admitted to “Hospital Regional Universitario de Málaga”, a 745 beds Spanish third level complex. From mid-April to mid-June, admissions declined to 40 patients, and from first of May to mid-June to eleven. From May, three COVID-19 admitted patients with HI were candidates to CCP, all them after readmission for persistence of symptomatic COVID-19.
Patient 1 was a 26-year-old male with X-linked agammaglobulinemia (XLA) diagnosed at months of life who came hospital on March 18th for receiving IVIg 30g. He had bronchiectasis in the middle lobe. From 2008, he had altered liver profile, thrombocytopenia, portal hypertension and splenomegaly, compatible with nodular regenerative hyperplasia.
From March 27th (day 1), patient began with fever, asthenia, cough, headache and diarrhea. On day 13, he came Emergency Room (ER). He had lymphopenia and a subtle right lung infiltrate. Under suspicion of COVID-19, though negative IgG/IgM ICA and SARS-CoV-2-RNA, hydroxychloroquine/azithromycin (H/A) and cefditoren were started. On day 16, he persisted with 39°C, baseline oxygen saturation (BOS) was 95%; and lymphocyte count (LC), C-reactive protein (CRP), ferritin and interleukin-6 (IL-6) values were 470/mm3, 25mg/l, 400ng/ml and 25.5pg/ml, respectively. D-dimer (D-D) and lactate dehydrogenase (LDH) were normal. Patient was admitted and COVID-19 was confirmed with a RT-PCR from a nasopharyngeal swab. He received ceftriaxone, corticosteroid boluses and IVIg 25 grams for three days but fever reappeared. On day 20, tocilizumab 600mg was administered. IL-6 concentration was 36.4pg/ml. CD19 lymphocytes were 0.1% (0), and IgG, A, M and E values 1.120/6/<5mg/dl and 10UI/ml, each. Complement C3/C4 was normal. Serum adenosine deaminase (ADA) rose to 78IU/L. On day 21, he was afebrile, LC fell to 210/mm3 and was randomized for remdesivir, stopped three days later as ALT peaked 200U/L. Dexamethasone was stopped simultaneously. At discharge, on day 26, he was afebrile and noticed a mild rash. BOS was 98%, LC rose to 710/mm3 and CRP normalized. RT-PCR was still positive. Right lung infiltrates improved and two small new round infiltrates developed in left lung.
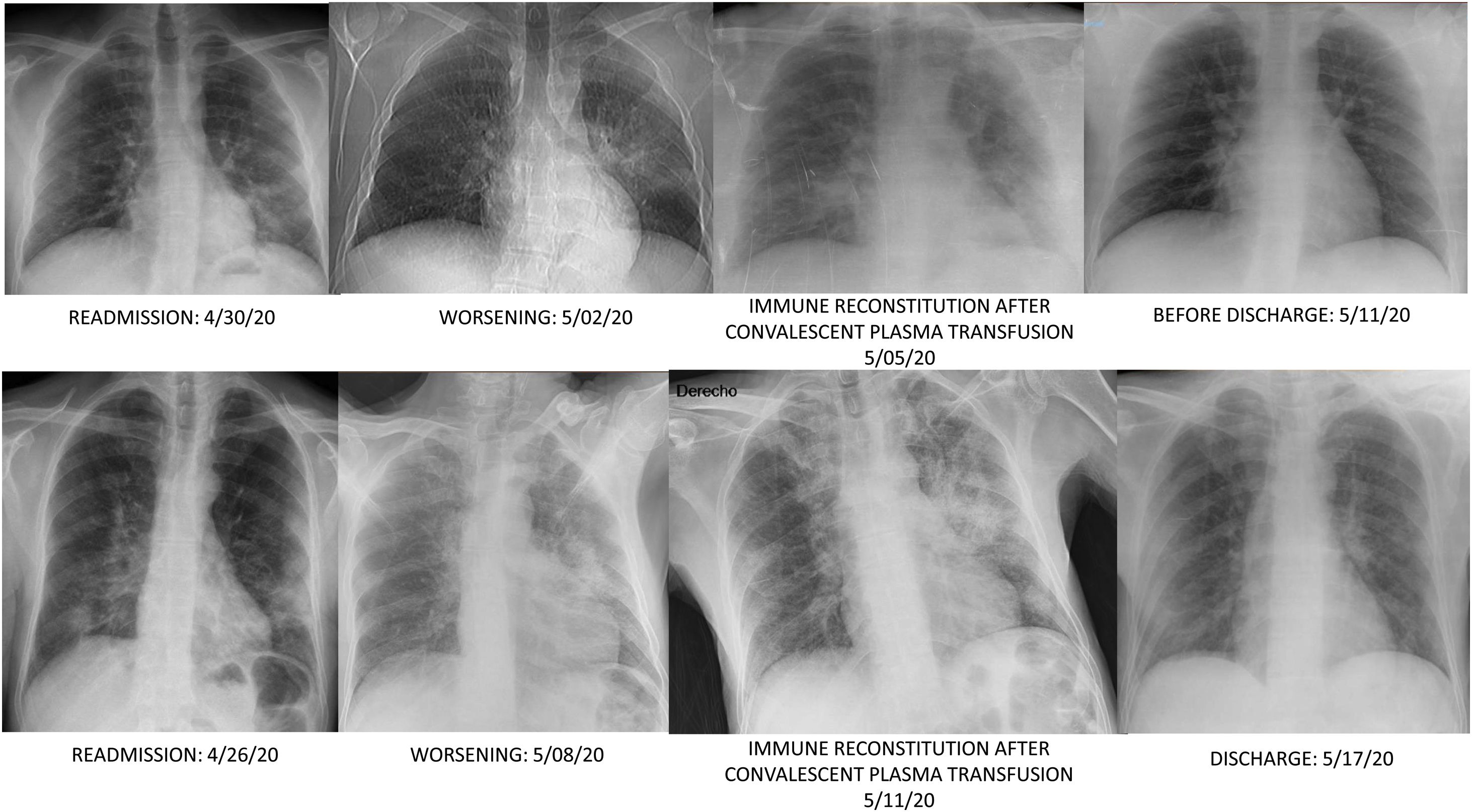
On day 35, patient was readmitted with dyspnea, cough and clear sputum. BOS was 92%. He had petechiae. LC was 470/mm3, and platelets count fell to 30,000/mm3. Meropenem was started. RT-PCR persisted positive in nasopharynx and sputum. Chest computed tomography (CT) showed patchy ground glass in upper lobes, pseudonodular consolidations with ground glass around, and consolidation with bronchogram in the left lower lobe. IgM+IgA/IgG (ELISA) antibodies persisted negative. Patient didn’t improve, so on day 39 received a first dose of 300ml of CCP (inactivated with Mirasol) matched by blood group (0+). CCP/cut-off index was 2.64. Before transfusion BOS was 95% and ADA peaked 177IU/L. On day 40, a fibrobronchoscopy (FBC) was done, and bronchoaspirate (BAS) and bronchoalveolar lavage (BAL) in the left lower lobe yielded abundant leukocytes; but complete microbiological determinations were negative excepting SARS-CoV-2 RNA positivity; and X-ray showed a new infiltrate in the right lower lobe (Fig. 1). Then BOS was 93% but late-night reached 96%. Since then, he didn’t require oxygen. IgM+IgA/IgG (ELISA) antibodies were negative/doubtful, each. On day 43, X-ray clearly improved. Trying to keep serum antibodies against SARS-Cov-2, a second dose of 300ml of CCP was transfused on day 45, without incidents. CCP/cut-off index was 5.42. On day 46, IgM+IgA/IgG (ELISA) antibodies were negative/positive respectively, but on day 50 IgM+IgA/IgG (ELISA) antibodies were negative/doubtful again. At discharge he was asymptomatic, BOS was 100%, petechiae were resolving and nasopharynx RT-PCR was negative. LC was 660/mm3, platelets count increased to 77,000/mm3 and ADA lowered to 52IU/L. Chest CT was normal.
Patient 2 was a 66-year-old male diagnosed of a primary cutaneous marginal zone B-cell lymphoma and of a mantle cell lymphoma, classic variant, affecting bone marrow on November 2018. After chemotherapy (VR-CAP) and complete remission, on November 7th 2019 received an autologous stem cell transplant (STC). His digestive tract was colonized by Candida glabrata. On January 14th 2020, subcutaneous rituximab 1400mg was reassumed, receiving last dose on March 11th 2020.
On April 5th (day 1), patient began with cough, scarce yellowish sputum, hyporexia, anosmia and ageusia. On day 9, he made 39°C, and was admitted. He was eupneic and BOS was 98%. LC, D-D, CRP, LDH and ferritin values were 350/mm3, 933ng/ml, 99mg/l, 241IU/L and 280ng/ml, respectively. COVID-19 ICA was negative and nasopharynx RT-PCR positive. Chest film disclosed a subpleural infiltrate in the upper right lobe resembling the CT “parallel pleura sign”. H/A were prescribed. On day 11, patient remained febrile, dyspneic and BOS fell to 92%. LDH and ferritin rose to 304IU/L and 456ng/ml, each. IL-6 level was 67.7pg/ml, receiving tocilizumab 400mg, and dexamethasone 40mg four days, rendering afebrile. On day 15, dyspnea improved and BOS was 96%, LC, D-D, CRP, LDH, ferritin values were 220/mm3, 574ng/ml, 10mg/l, 335IU/L and 448ng/ml, each. Lung infiltrate improved and patient was discharged.
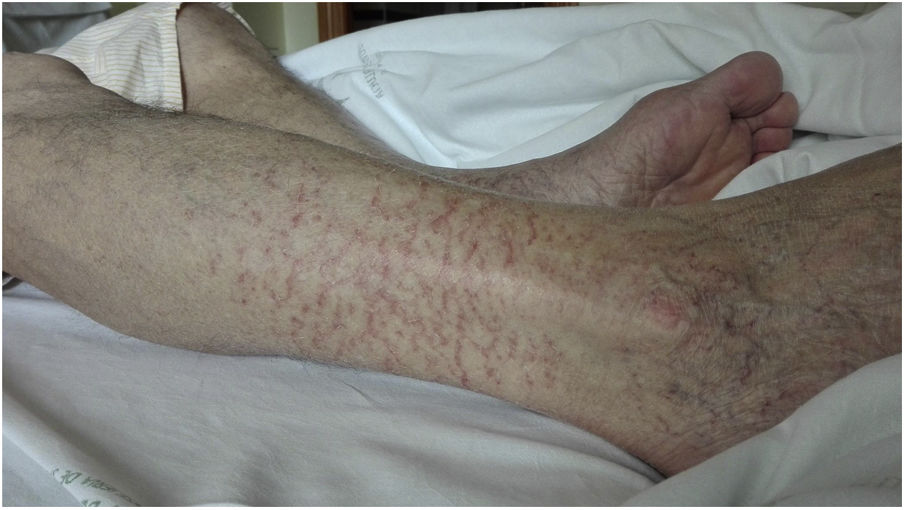
On day 22, patient was readmitted with dysthermia, dyspnea and scarce yellowish sputum. BOS was 93%. LC, D-D, CRP, LDH, ferritin and IL-6 values were 300/mm3, 2,362ng/ml, <2.9mg/l, 368IU/L, 477ng/ml and 767.4pg/ml, respectively. CD19 lymphocytes were 0% and IgG, A and M values 268/12/<5mg/dl, each. Complement C3/C4 was normal. Serum ADA rose to 71IU/L. Dexamethasone 6mg, cefepime and liposomal amphotericin B (LAmB) 250mg were started. LAmB was stopped two days later after worsening renal function. COVID-19 ICA was negative, and nasopharynx RT-PCR positive. Chest CT showed bilateral subpleural patchy ground glass infiltrates and excluded pulmonary embolism. On day 24, a FBC was done. Microbiology of BAS and BAL was negative, excepting isolation of Candida albicans/glabrata and SARS-CoV-2 RNA positivity. BAL cytology showed 50% macrophages, 40% neutrophils and 10% lymphocytes. On day 28, infiltrates improved and dexamethasone was stopped. On day 31, low grade-fever reappeared, BOS was 94% and an left lower lobe infiltrate reappeared. Cefepime was changed to voriconazole and meropenem. So on day 36 a first dose of 300ml of CCP, matched by blood group (A+), was transfused. CCP/cut-off index was 3.95. Eight hours later, he made 38.5°C and BOS fell to 89%. LC, D-D, CRP, LDH, ferritin and IL-6 values were 180/mm3, 1,276ng/ml, 28.5mg/l, 266IU/L, 439ng/ml and 1859pg/ml, respectively. ADA lowered to 38.3IU/L. IgM+IgA/IgG (ELISA) antibodies remained negative. Blood cultures were negative and chest film slightly worsened (Fig. 1). On day 37, patient was afebrile and BOS reached 93%. Antimicrobials were stopped. On day 38, BOS was 97%. On day 40, lung infiltrates were clearer and small vasculitis lesions appeared over ankles suggesting immune-complex deposition (Fig. 2). A second dose of 300ml of CCP from the same donation was transfused, without clinical incidents. On day 41, LC, D-D, CRP, LDH and ferritin values were 390/mm3, 4006ng/ml, 4.6mg/l, 234IU/L and 392ng/ml each. Serum ADA was low, 41.2IU/L. IgM+IgA/IgG (ELISA) antibodies persisted negative again. Left lung infiltrate slightly worsened again. On day 43, patient was discharged. Expectoration was scarce, BOS 100%, and chest film normal. Enoxaparin was kept, and on day 51, LC reached 780/mm3, D-D lowered to 1606ng/ml, and other parameters normalized, including IL-6. RT-PCR was negative in sputum.
Patient 3 was a 62 year-old-male diagnosed of a follicular non-Hodgkin lymphoma grade 3B (WHO) on August 2019. Though very intense bone marrow uptake in PET-TAC, biopsy didn’t show lymphomatous infiltration. At diagnosis, patient had severe hypogammaglobulinemia, and IgG, A and M serum concentrations were 255, 85 and 52mg/dl, respectively. In routine medical check-ups on February 2016, IgG/A/M values were 630/97/63mg/dl, moving to 447/43/19 on December 2018. On September 2019 5th, received first R-CHOP, and eighteen days later was admitted with cytomegalovirus pneumonia, resolved with ganciclovir. On October 2019 31st, IgG, A and M values were 81/12/<5mg/dl, receiving IVIg, with fever after infusion as side effect. On January 2020 24th, finished sixth R-CHOP, with metabolic complete remission at third and sixth cycle (Deauville 1). On March 13th, IgG, A and M values were 235/<6/<5mg/dl, and CD19 lymphocytes were 0%. Subcutaneous rituximab was administered, and on April 3rd began mobilization with VP16 and daily SC G-CSF.
On April 9th (day 1), patient began with dry cough. On day 5, patient made 38.9°C and on day 7, was admitted for canalizing central line and collecting peripheral blood hematopoietic precursors for autologous stem cell transplant (SCT), being transferred to the Infectious Diseases Department. BOS was 92%. LC was 1940/mm3. On day 11, BOS was 97% and LC, D-D, CRP, LDH and ferritin values were 880/mm3, 1278ng/ml, 56mg/l, 378IU/L and 1439ng/ml, each. Serum IL-6 was 34.7pg/ml. COVID-19 nasopharynx RT-PCR was positive. Chest film showed subtle patchy infiltrates, mainly in left hemithorax. After treatment with H/A, cefepime and enoxaparin, cough improved, fever remitted and BOS reached 98%, being discharged on day 14. LC, D-D, CRP, LDH and ferritin values were 1910/mm3, 1953ng/ml, 42mg/l, 180IU/L and 1144ng/ml, respectively.
On day 55, patient referred intermittent evening fever, asthenia and cough without dyspnea nor desaturations from discharge, being readmitted. COVID-19 nasopharyngeal RT-PCR persisted positive on days 30, 37 and 48. He made 37.4°C and BOS was 98%. LC, D-D, CRP, LDH and ferritin values were 1520/mm3, 613ng/ml, 48mg/l, 282IU/L and 886ng/ml, each. Serum ADA and IL-6 was 51.3IU/L and 15.8pg/ml, respectively. CD 19 lymphocytes were 0.1% (2/mm3) and IgG, A and M values 136/10/<5mg/dl, each. Complement C3/C4 was normal. IgM+IgA/IgG (ELISA) antibodies were negative and SARS-CoV-2 nasopharynx RT-PCR was positive. X-ray showed an interstitial pattern in upper left lobe, lingula and apical segment of right lower lobe. IVIg 30g were administered for hypogammaglobulinemia. He made fever and BOS lowered to 95%. On day 56, he was afebrile and BOS was 99%. A dose of 300ml of CCP, matched by blood group (A+) was transfused. CCP/cut-off index was 3.69. Though acetaminophen, he made 37.4°C later and BOS lowered to 96%. On day 57, he felt good, afebrile and BOS was 98%, being discharged. On day 63, LC, D-D and LDH values were 3900/mm3, <2.9mg/l and 261IU/L, respectively. COVID-19 nasopharynx RT-PCR negativized.
DiscussionWhen SARS-CoV-2 binds to a cell through the host receptors ACE-2 and TMPRSS2, it replicates inside the cell and following the release of virus particles, virus promotes host cell entry in pyroptosis. Different signals activate the innate immune response in the lungs, mainly mediated by granulocytes, antigen presenting cells and pro-inflammatory macrophages. This system is coordinated and supported by an adaptive immune response, represented by CD4+ T cells, CD8+ T cells, Tregs and B cells. The activation and proliferation of CD4+ T cells fosters CD8+ T cells, crucial for antiviral responses through the induction of cell death. Likewise, B cells secrete antibodies to neutralize the virus or induce the lysis of infected cells by complement activation of antibody-dependent cellular cytotoxicity (mediated by natural killer (NK) cells).4 The first response to a novel virus is typically characterized by the cooperation between NK cells and natural antibodies, key components of innate immune system. They contain infection whilst adaptive immune response develop. NK cell counts are related with the evolution of COVID-19. Asymptomatic individuals have the highest NK cell count, and a monocyte to NK ratio under 1. Patients with mild disease have a monocyte to NK ratio slightly over 1, and patients with severe disease had a quite higher ratio, secondary to a an increase of monocyte count and a severe NK depletion. This pattern was not incidental and persisted during all the clinical course of the disease, so it points to the individual immune response of every patient to SARS-CoV-2.5 However, a coordinated early adaptive immune response with generation of SARS-CoV-2 specific CD4+ and CD8+ T cells and neutralizing antibodies is associated with a less severe outomes.6 The majority of infected patients mount a SARS-CoV-2 specific antibody response during the acute phase of illness. For IgM, mean or median time to seroconversion ranges from 4 to 14 days post symptom onset; for IgM, from 12 to 15 days. Majority of patients develop detectable neutralizing antibodies between 7 and 15 days following disease onset, increasing over days 14–22 before plateauing.7 Neutralizing antibodies are crucial against SARS-CoV-2. Delayed seroconversion kinetics correlates with impaired viral control in deceased patients, and neutralizing antibodies generation prior to 14 days of disease onset is a key factor for recovery.8 In fact, between hospitalized patients not receiving mechanical ventilation treated with CCP, mortality was lower in those who received plasma with higher titers of SARS-CoV-2 antibodies. Though quantification of IgG was determined by CLIA and does not differentiate binding IgG antibodies from virus-neutralizing IgG antibodies, a strong correlation has been shown between the amount of antispike protein IgG and in vitro virus neutralization.9 Recently, a randomized clinical trial has shown that early transfusion of high-titer CCP prevents severe COVID-19 in older adults. There was a dose-dependent IgG effect in convalescent plasma infusions. Plasma with IgG titers of 1:3200 or higher reduced the risk of severe respiratory disease by 73%. This directly implicates antibodies as the active therapeutic ingredient in convalescent plasma.10 Finally, T cells are important regulators of cellular and antibody-mediated immunity. They recognize antigens from pathogen proteins and differentiate into a range of effector cell types, including “follicular helper” CD4+ T cells that induce B cells to produce high-affinity antibodies capable of neutralizing the pathogen and cytolytic CD8+ T cells that kill pathogen-infected cells.11
So, as control of COVID-19 relies completely on immune system, patients with an enabled immunity, could have a worse prognosis, or a prolonged clinical course and shedding of the virus. In this setting, CCP could be a reasonable option for patients with a defective HI.12
First reported patients with COVID-19 and primary immunodeficiencies were two cases with XLA who were symptomatic and required admission for 10–22 days13; a case with common variable immunodeficiency (CVID) and Sjogren's disease on hydroxychloroquine, who required invasive mechanical ventilation14; and a case with very low humoral response to COVID-19, associated to a mutation in TRNT1 (tRNA nucleotidyl transferase) gene, who relapsed and had a prolonged course.15 In a case series from USA, most prevalent primary immunodeficiencies reported with COVID-19 were CVID, followed by XLA, hypogammaglobulinemia, IgA-IgG2 deficiency, interferon gamma receptor 2 deficiency and X-linked hyper-IgM syndrome.16 COVID-19 is a serious problem in patients with primary and secondary immunodeficiency. In a registry from UK, 53.3% (32/60) of patients with primary immunodeficiencies and COVID-19 were hospitalized and inpatient mortality was 37.5% (12/32); patients with secondary immunodeficiencies did worse: 75.8% were hospitalized (25/33) and inpatient mortality was 44% (11/25). CCP use is not reported in this paper.17
First patient with COVID-19 and XLA cured with CCP was symptomatic for 37 days. After transfusion of 200ml of CCP, fever disappeared, RT-PCR negativized and a week later chest CT normalized, being discharged.18 Three other XLA patients with prolonged COVID-19 were cured with 400ml of CCP. First had low antibody titers after transfusion, second had fever after transfusion, and third was discharged a day after.19 A patient with persistent COVID-19 and CVID cured after receiving CCP16; however, another patient with COVID-19 and CVID died 24 days after receiving CCP and high-dose IVIG, but clinical condition was critical when CCP was administered. Dose of CCP was not mentioned.20 Another patient with COVID-19 and CVID has been reported in a case-series from France.21 Recently, a patient with hypogammaglobulinemia, renal transplantation and history of lymphoma died of COVID-19 though been treated with CCP16 (Table 1).
Patients with primary humoral immunodeficiency and covid-19 treated with convalescent plasma.
| Case | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 26 | 39 | 10 | 24 | 40 | 65 | 42 | N.R. | 39 |
| Gender (M/F) | M | M | M | M | M | F | M | N.R. | F |
| Date of administration of first convalescent plasma | 04-May-2020 | 6-May-2020 | N.R. | N.R. | N.R. | N.R. | May-2020 | N.R: | N.R. |
| Amount of plasma (ml) | 300 | 200 | 200 | 400 | 400 | N.R. | N.R. | 400 | N.R. |
| Date of administration of second convalescent plasma | 10-may-2020 | – | Next day | – | – | – | – | Next day | – |
| Amount of plasma (ml) | 300 | – | 200 | – | – | – | – | 400 | – |
| Disease responsible of humoral immunosuppression | XLA | XLA | XLA | XLA | XLA | CVID | CVID | CVID | Hypogammaglobulinemia |
| Adverse reactions | Mild decrease BOS | – | – | Fever | – | – | – | – | – |
| Days from first symptoms to plasma administration | 38 | 37 | 32 | 21 | 44 | 41 | 9 | – | 36 |
| Days from first plasma administration to discharge/exitus | 10 | 7 | 6 | 3 | 1 | N.R. | 25 | – | N.R. |
| Outcome | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable | Exitus | N.R. | Exitus |
| Reference | Current case 1 | Ref. 18 | Ref. 19 | Ref. 19 | Ref. 19 | Ref.16 | Ref.20 | Ref. 21 | Ref. 16 |
Gender: M=Male, F=Female; XLA: X-linked agammaglobulinemia; CVID: common variable immunodeficiency; N.R.: not reported; BOS: Baseline oxygen saturation.
In a British series of patients with hematological malignancies, mortality of COVID-19 rose to 40%. Strikingly, 54% of patients had pre-existing hypogammaglobulinemia at baseline.22 Underlying disease and anti-tumor therapies induce immunodeficiency in hematological malignancies patients, involving B-cell dysfunction and hypogammaglobulinemia, numerical and functional abnormalities in neutrophils, dendritic cells, T cells, and dysfunctional natural killer cells.23
Rituximab, a monoclonal anti-CD20 antibody, reacts with the CD20 antigen, located on pre-B and mature B lymphocytes. Rituximab can be detected in the serum for many months after the dose of drug. Rituximab administration induces a rapid elimination of circulating B cells. Mechanisms implied are complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity and stimulation of the apoptotic pathway.24 So depletion of B lymphocytes can impede an adequate antibody response to SARS-CoV-2. As B cells are effective antigen-presenting cells and activators of T cells,25 lack of them can induce a defective cellular immune response too. Besides, rituximab can induce baseline hypogammaglobulinemia. In a series of 243 patients receiving rituximab for multi-system autoimmune diseases, with median follow up of 42 months, 56% had IgG hypogammaglobulinemia (<7g/L) during follow up and IgM was under 0.3g/L in 58% of patients.26 And in a cohort of patients who received rituximab, among those who had their IgG levels determined both before and after rituximab use, 19% with normal IgG levels before rituximab therapy developed mild to severe hypogammaglobulinemia after rituximab, 23% with mild hypogammaglobulinemia before rituximab developed moderate to severe hypogammaglobulinemia after rituximab, and 21% with moderate hypogammaglobulinemia before rituximab went on to develop severe hypogammaglobulinemia following rituximab use.27 Rituximab is widely used in hematological patients affected with non-Hodgkin lymphoma, and in patients with diverse autoimmune or neuro-immunological diseases. However, some authors consider that hyperinflammation-associated organ failure during COVID-19 may be less pronounced in hematological malignancies by the own hematological condition itself or by treatment-related immunosuppression as treatment with rituximab.28
In a French series, three patients ongoing rituximab maintenance for diffuse large B-cell lymphoma, nodular lymphocyte-predominant Hodgkin lymphoma and Waldenström macroglobulinemia, didn’t develop acute respiratory distress syndrome nor required invasive mechanical ventilation. First one needed anakinra and the third patient stood on dexamethasone.29 However, clinical course can be prolonged in these patients28,30 or deathful.31 A death associated to reinfection of COVID-19 two months after a first episode has been described in a patient with Waldenström macroglobulinemia on rituximab.32
CCP has been used successfully in COVID-19 hematological malignancy patients on rituximab. A patient with B-cell acute lymphoblastic leukemia treated with allogeneic SCT before, and chronic graft-versus-host disease (cGVHD), treated with steroids and weekly IV rituximab, cured after receiving two transfusions of 200ml of CCP within a lapse of 9 days.33 Another patient with an orbital and meningeal marginal zone lymphoma and Sjögren's syndrome on bendamustine and rituximab, cured after two consecutive doses of 400ml of CCP. After first dose, antibodies against SARS-CoV-2 positivized and a week later RT-PCR was negative.34 A patient with a stage IVE follicular lymphoma, treated with bendamustine and rituximab 2 years before, ongoing rituximab maintenance, was readmitted in ICU. Three days after receiving 200ml of CCP, on day thirty-one of evolution, was afebrile, and biomarkers and chest film normalized.35 Another patient with a mantle cell lymphoma who received four months before an autologous SCT and was on rituximab, had a long hospital stay with COVID-19 and cured, having received CCP. SARS-CoV-2 was positive al discharge. Dose of CCP was not reported.36 A patient with non-Hodgkin lymphoma in remission while on maintenance therapy with Obinutuzumab, was symptomatic with COVID-19 for eighty-eight days, curing after transfusion of 200ml of CCP.37 Another patient with follicular lymphoma in remission while on Obinutuzumab, had a recurrent and protracted COVID-19 for eighty-six days, curing after receiving CCP.38 In a case-series from France, seventeen B-cell depleted consecutive patients with protracted COVID-19 were treated with CCP. Fifteen had hematological malignancies, most of them receiving rituximab. Median serum immunoglobulin levels were 350mg/dl. Median evolution of clinical picture was 56 days. All them were transfused with 400ml two consecutive days. All survived but one. Median stay from transfusion to discharge was 7 days.21 CCP has been useful in patients with COVID-19 and chronic lymphocytic leukemia after rituximab/bendamustine,39 B-cell acute lymphoblastic leukemia on rituximab, cytarabine and dasatinib40 (Table 2) or maintenance therapy with 6-Mercaptopurine and Methotrexate,41 and severe aplastic anemia.42 Prolonged shedding of SARS-CoV-2 though receiving CCP has been described in a patient with mixed cellularity classical Hodgkin lymphoma 4 years before, peripheral T-cell lymphoma 17 months before and an autologous SCT 6 months before, in partial remission, with hypogammaglobulinemia. As persisted symptomatic for COVID-19, he received CCP. Three days later, patient became afebrile for a month, and anti-SARS-CoV-2 IgG was positive from then. However, RT-PCR persisted positive, though CT clearly increased 17 days later.43
Patients with anti CD20 induced humoral immunosuppression and COVID-19 treated with convalescent plasma.
| Case | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9-23 | Patient 24* | Patient 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 66 | 62 | 53 | 76 | 54 | 40-60 | 63 | 53 | 58 [35–77] | 76 | 55 |
| Gender (M/F) | M | M | F | F | M | N.R. | F | F | 5F/12M | F | F |
| Date of administration of first convalescent plasma | 10-may-2020 | 3-jun-2020 | 27-apr-2020 | 14-may-2020 | 15-apr-2020 | N.R. | Jun-2020 | 20-jun-2020 | N.R. | 12-jun-2020 | 27-jun-2020. |
| Amount of plasma (ml) | 300 | 300 | 200 | 400 | 200 | N.R. | 200 | N.R. | 400 | 200 | N.R. |
| Date of administration of second convalescent plasma | 14-may-2020 | – | 6-may-2020 | 15-may-2020 | – | – | – | 5-jul-2020 | Next day | 13-jun-2020 | – |
| Amount of plasma (ml) | 300 | – | 200 | 400 | – | – | – | N.R. | 400 | 200 | – |
| Disease responsible of humoral immunosuppression | Mantle cell lymphoma, autologous SCT, ongoing rituximab maintenance | Follicular lymphoma, ongoing rituximab maintenance | B-cell acute lymphoblastic leukemia, allogeneic SCT, cGVHD ongoing rituximab maintenance | Marginal zone lymphoma, ongoing rituximab maintenance | Follicular lymphoma, ongoing rituximab maintenance | Mantle cell lymphoma, autologous SCT, ongoing rituximab maintenance | Non-Hodgkin lymphoma, ongoing obinutuzumab maintenance | Follicular lymphoma, ongoing obinutuzumab maintenance | 15 Hematological malignancies1 Multiple Sclerosis1 CVID | Cjhronic lymphocytic leukemia, treated with rituximab-bendamustine | B-cell acute lymphoblastic leukemia, treated with rituximab, cytarabine and dasatinib |
| Adverse reactions | Mild decrease BOS, Fever | Mild decrease BOS | – | No | – | – | Mild decrease BOS | – | – | – | – |
| Days from first symptoms to plasma administration | 35 | 57 | 4 | 50 | 32 | 34 | 88 | 90 | 56 [7–83] | 66 | 80 |
| Days from first plasma administration to discharge/exitus | 7 | 1 | 11 | 19 | 7 | 24 | 2 | 2 | 7 [2–14] | 7 | Few days |
| Outcome | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable | 16 Favourable/1 Exitus | Favourable | Favourable |
| Reference | Current case 2 | Current case 3 | Ref. 33 | Ref. 34 | Ref. 35 | Ref. 36 | Ref. 37 | Ref. 38 | Ref. 21 | Ref. 39 | Ref. 40 |
Gender: M=Male, F=Female; N.R.: Not reported; STC: Stem cell transplant; cGVHD: Chronic Graf-versus-host disease; BOS: Baseline oxygen saturation.
Patients 9–23: Age, Days from first symptoms to plasma administration, Days from first plasma administration to discharge/exitus=median, range between brackets.
Patient 24 received 4 consecutive doses of 200ml of CCP from 12th to 15th of June 2020.
Our patients eradicated COVID-19 clearly after transfusion of CCP. Surprisingly, the day after administration of CCP, COVID-19 IgG levels (ELISA) were dubious or negative. We hypothesize consumption of CCP antibodies in lung tissue against SARS-CoV-2. This lack of neutralizing antibody titers in serum after transfusion of CCP has been described in critically ill COVID-19 patients. All had persistently positive nucleic acid tests before CCP, and negativized SARS-CoV-2 RNA soon after.44 Fever, decrease of BOS and new or worsening infiltrates suggest immune reconstitution. The second dose of 300ml of CCP administered to patient 1 rendered serum antibodies that waned in a week and no antibodies for patient 2. That is why we did not give a second transfusion to patient 3. Then, plasma antibodies are short-lived probably by consumption of them. Clinical improvement was associated to virus eradication.
Patients with rheumatic inflammatory diseases on treatment with rituximab infected by SARS-CoV-2 have been described too. Most of them suffered rheumatoid arthritis, ANCA-associated vasculitis or systemic sclerosis. In the Germany National Registry for patients with inflammatory rheumatic diseases infected with SARS-CoV-2, 67% of those receiving rituximab required hospitalization.45 And in two Spanish cohorts of patients with rheumatic diseases and COVID-19, all the seven patients receiving rituximab required hospitalization and one died46 and eight of thirteen required hospitalization and three died.47 Patients with neuro-immunological diseases under rituximab with COVID-19 have a more protracted and severe disease,48 sometimes requiring mechanical ventilation.49 A patient with multiple sclerosis treated with CCP has been reported.21
Most common primary cellular immunodeficiencies are severe combined immunodeficiency (SCID) and CVID. In SCID, T lymphocytes are lacking and B lymphocytes function fails,50 so CCP could be useful in this disorder. In CVID, anti-viral T cell immunity is not relevantly impaired in most patients,51 but being humoral response defective, CCP can benefit patients. Main acquired cellular immunodeficiency is related with HIV infection. People with HIV have a higher risk for severe COVID-19 because of immunodeficiency, comorbidities and/or social inequities; and worse COVID-19 outcomes. Prognosis is worse for patients with CD4+ cell counts under 200/mm3.52 It is unknown if patient with low CD4+ cell counts could elaborate a good cellular and humoral response to COVID-19. At present CCP has been transfused successfully to an HIV patient with a lymphoma under treatment with obinutuzumab with less than 50 CD4+ cells and hypogammaglobulinemia53; and in a series of eleven transplant recipients with HIV infected with SARS-CoV-2, two were transfused with CCP: one survived and the other died.54 Patients with solid organ transplantation (SOT) are treated usually with calcineurin inhibitors, purine synthesis inhibitors and corticosteroids. Calcineurin inhibitors inhibit activation and proliferation or T cells, synthesis of cytotoxic T lymphocytes, and development and differentiation of B cells. Purine synthesis inhibitors like mycophenolic acid derivatives inhibit proliferation of T and B lymphocytes. Corticosteroids inhibit synthesis of cytokines activating T and B lymphocytes.55 Patients with SOT and COVID-19 have higher mortality and need of invasive mechanical ventilation than non-SOT recipients.56 From the beginning of pandemic, kidney transplant recipients with mild COVID-19 have continued calcineurin inhibitors and prescribed doses of corticosteroids stopping antiproliferative drugs, and patients with severe COVID-19, have stopped all immunosuppressant drugs increasing corticosteroids dose.57 A multi-center study on liver transplant recipients with COVID-19 has shown that keeping tacrolimus at the usual dose is associated with a better survival.58 Though prolonged viral shedding has been described in kidney transplant recipients with COVID-19,59 patients use to harbour antibodies against SARS-CoV-2 by day 15.60 So, CCP could not be useful for patients infected for more than 15 days. Experience with CCP in patients with COVID-19 and SOT is sparse. In a systematic review of COVID-19 in SOT recipients, CCP was used in 33 patients. Mortality was 12.9% (4/31 patients).61 This percentage is lower than global mortality by COVID-19 in SOT recipients (18.6%)61 and in transplant recipients (27%).62
Concerns of CCP are transfusion-associated circulatory overload (TACO), an increase of the inflammatory and prothrombotic status induced by SARS-CoV-2 mediated by complement proteins and coagulation factors infusion, and antibody-dependent enhancement of the virus. It occurs when antibodies levels are insufficient to fully block viral entry but are sufficient to opsonize virus. This can facilitate viral entry into cells and can enhance viral toxicity, worsening inflammation.63 An analysis of safety metrics after transfusion of ABO-compatible human CCP in 20,000 hospitalized adults with or at high risk of progression to severe or life-threatening COVID-19 in USA rendered 78 transfusion reactions. Related mortality within four hours of transfusion was 0.05%, and TACO, TRALI and severe allergic transfusion reactions happened in 0.18, 0.10 and 0.10% of patients, respectively.64 Volumes of 300ml supposed no TACO for our patients. Though 300ml of convalescent plasma equates to 3g of IgG,65 IgG levels didn’t increase after CCP, but IgA/IgM increased slightly. There was no consumption of complement proteins, as previously described in COVID-19.66 In our experience, 300ml of convalescent plasma was enough. Dose must be body weight adjusted, and for people around 70kg, 210ml can be sufficient.67
Regarding diagnosis, in these patients neither ICA nor ELISA serology is useful, given B-cells depletion at baseline; moreover, seronegative condition can be used as a marker prompting for CCP administration in patients with HI and COVID-19. Studies analyzing antibody production in patients suffering other hematological conditions or malignancies are needed, as this can be absent, insufficient or anomalous. We are not aware of any clinical trial running now in this specific population,68 and paradoxically these patients are excluded from most clinical trials using CCP. It seems reasonable to offer CCP for those patients with hematological conditions if there is suspicion of any kind of HI. Persistent SARS-CoV-2 RT-PCR is typical in these patients till receiving CCP.
Serum adenosine deaminase (ADA) was increased in our patients. Infections activating monocytes/macrophages can increase serum adenosine deaminase (ADA), particularly ADA2 isoenzyme, as it has been shown in HIV.69 ADA may play a role in COVID-19 pathogenesis. ADA could have a non-enzymatic role competing the virus for binding to the CD26 receptor, preventing entry and spread of the virus; and an enzymatic role, metabolizing adenosine to inosine. As immunosuppression by adenosine can hinder the elimination of the virus, adenosine metabolism could be of benefit.70 From first transfusion, ADA normalized in our patients, and could be a marker of COVID-19 severity.
In patient 2, cytology of BAL rendered mainly macrophages. This has been described in COVID-19, and lung macrophages probably develop from blood monocytes.71 This finding could be a diagnostic clue in patients with COVID-19 absent antibodies and RT-PCR SARS-CoV-2 negative.
Summarizing reported patients with HI and COVID-19 treated with CCP (Tables 1 and 2), it must be highlighted that patients with a favourable outcome improved after one or two doses of CCP, every one ranging from 200 to 400ml. Most remarkable, evolution of COVID-19 before CCP lasted even 88 days,37 so never is too late for transfusing CCP. However, in severe COVID-19 patients, CCP could not reverse clinical picture nor avoid death of the patient. Most reported patients negativized SARS-CoV-2 quickly and could leave the hospital.
In conclusion, CCP is useful for patients with agammaglobulinemia or hypogammaglobulinemia and COVID-19, helping to eradicate SARS-CoV-2 and avoiding relapses and chronicity. This treatment should be a priority in this setting. A 300ml transfusion can be enough. Quantification of serum immunoglobulins, B lymphocytes and determination of COVID-19 antibodies at initial evaluation could detect patient candidate for CCP early. Patients with hematological malignancies should receive special consideration.
Conflict of interestsThe authors declare that they have no conflict of interests.
To all colleagues implicated in caring COVID-19, patients and their families.