SARS-CoV-2 infection patients face infectious complications, including fungal infections. COVID-19-Associated Pulmonary Aspergillosis (CAPA) is linked to SARS-CoV-2 damage, corticosteroids, and pulmonary diseases. Diagnostic uncertainties persist, and this study aims to contribute evidence on CAPA risk factors, diagnostics, and prognosis.
MethodsA retrospective case–control study focused on critically ill COVID-19 patients with CAPA between March 2020 and December 2022 in a second-level hospital. Variables included demographic and medical history, infection course, treatments, complications, and outcomes.
Results27 CAPA cases and 56 controls were collected. CAPA prevalence was 5.1% considering adapted criteria. CAPA cases were associated with cardiovascular risk factors, autoimmune diseases, chronic corticosteroid therapy, and other immunosuppressants, RRT, ECMO, cumulative corticosteroid dose, direct ICU admission, and invasive mechanical ventilation. They exhibited higher RALE and APACHE-II scores, direct ICU admission, and more invasive ventilatory support. CAPA patients had a higher risk of mortality at 120 days. The CAPA score demonstrated sensitivity and specificity in predicting CAPA risk.
ConclusionsThere is a high mortality rate at 120 days among cases (67%). Classical risk factors and other new ones, such as the use of ECMO, autoimmune diseases, or direct admission to the ICU, have been postulated. The accumulated dose of steroids (>800mg of metilprednisolone) is one of the key risk factors in the development of CAPA. The CAPA score is a useful tool to define which patients should be monitored closely, although more studies are still needed.
Los pacientes infectados con SARS-CoV-2 padecen complicaciones infecciosas, incluidas las infecciones fúngicas. La aspergilosis pulmonar asociada a COVID-19 (CAPA) está relacionada con el daño causado por el SARS-CoV-2, los corticosteroides y las enfermedades pulmonares. Persisten incertidumbres diagnósticas, y este estudio tiene como objetivo contribuir a la evidencia sobre los factores de riesgo, el diagnóstico y el pronóstico.
MétodosEstudio retrospectivo de casos y controles en pacientes críticos con neumonía COVID-19 entre marzo de 2020 y diciembre de 2022 en un hospital de segundo nivel. Las variables incluyeron antecedentes demográficos y médicos, curso de la infección, tratamientos, complicaciones y resultados.
ResultadosSe recopilaron 27 casos de CAPA y 56 controles. La prevalencia de CAPA fue del 5,1% considerando los criterios adaptados. Los casos de CAPA estuvieron asociados con factores de riesgo cardiovascular, enfermedades autoinmunes, terapia crónica con corticosteroides y otros inmunosupresores, RRT, ECMO, dosis acumulativa de corticosteroides, ingreso directo a la UCI y ventilación mecánica invasiva. Exhibieron puntajes más altos de RALE y APACHE-II, ingreso directo en UCI y soporte ventilatorio invasivo. Los pacientes con CAPA tuvieron un mayor riesgo de mortalidad a los 120días. La puntuación en el CAPA score demostró sensibilidad y especificidad para predecir el riesgo de CAPA.
ConclusionesExiste una elevada mortalidad a los 120días entre los casos (67%). Los factores de riesgo clásicos y otros nuevos, como el uso de ECMO, enfermedades autoinmunes o ingreso directo en UCI, se han postulado como potenciales factores de riesgo. La dosis acumulada de esteroides (>800mg de metilprednisolona) es uno de los factores de riesgo clave en el desarrollo de CAPA. El CAPA score es una herramienta útil para definir qué pacientes deben ser vigilados de forma especial, aunque faltan estudios para completar su validación.
Patients with moderate to severe SARS-CoV-2 admitted to hospitals commonly experienced a variety of complications arising from the infection and hospital admission.1 Fungal infection stood out, including invasive pulmonary aspergillosis (IPA).1–3 Various prevalences have been reported regarding fungal co-infection with SARS-CoV-2, however, there are still many uncertainties regarding definitions, risk factors and management.4–8 IPA in the context of a viral infection has been also described related to with Influenza, termed Influenza-Associated Pulmonary Aspergillosis (IAPA).7–11 The pathogenesis of IPA in viral infections is based on the direct damage caused to the respiratory epithelium, promoting bronchial and pulmonary invasion.11,12 SARS-CoV-2 damage directedly the respiratory epithelium, coupled with the hyperinflammatory syndrome and the immunoparalysis.13–15
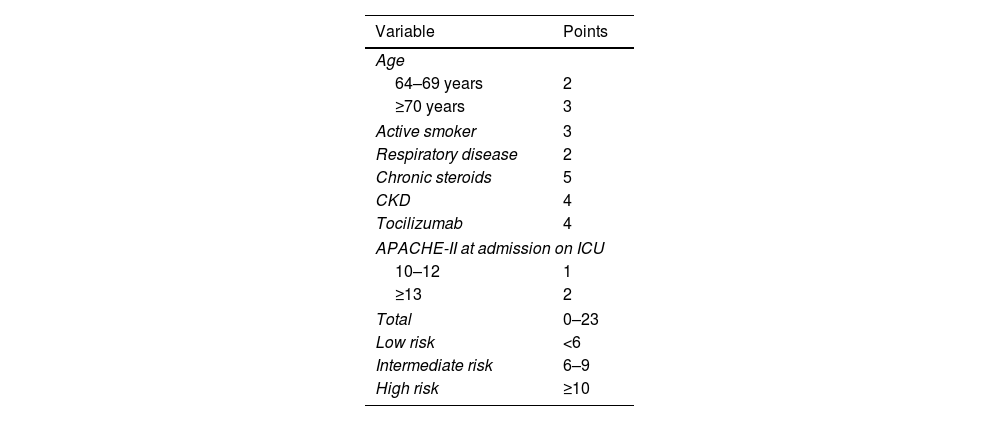
Various studies published have investigated the risk factors for developing CAPA.1,3–7,16–22 The prevalence of CAPA among ICU patients varies among the studies due to the different criteria used over the years.6 It is noteworthy that the definitions proposed by Köhler et al.8 are the only ones that consider the subgroup of “possible CAPA”. Various risk factors have been proposed over the years for the development of CAPA.7,11,18,22,23,25 In 2022, a new tool was developed to stratify patients at higher risk of developing CAPA (Appendix 1).26
Currently, there are uncertainties regarding the risk factors, diagnosis, and prevention of CAPA. The present study contributes with additional evidence on the risk factors and diagnostic tools required for the accurate identification of these patients. The primary objective of our study is to establish the risk factors associated with CAPA development in the context of critically ill patients with severe COVID-19 pneumonia. Furthermore, we will analyze the prognosis of CAPA patients and CAPA score applied in our sample.
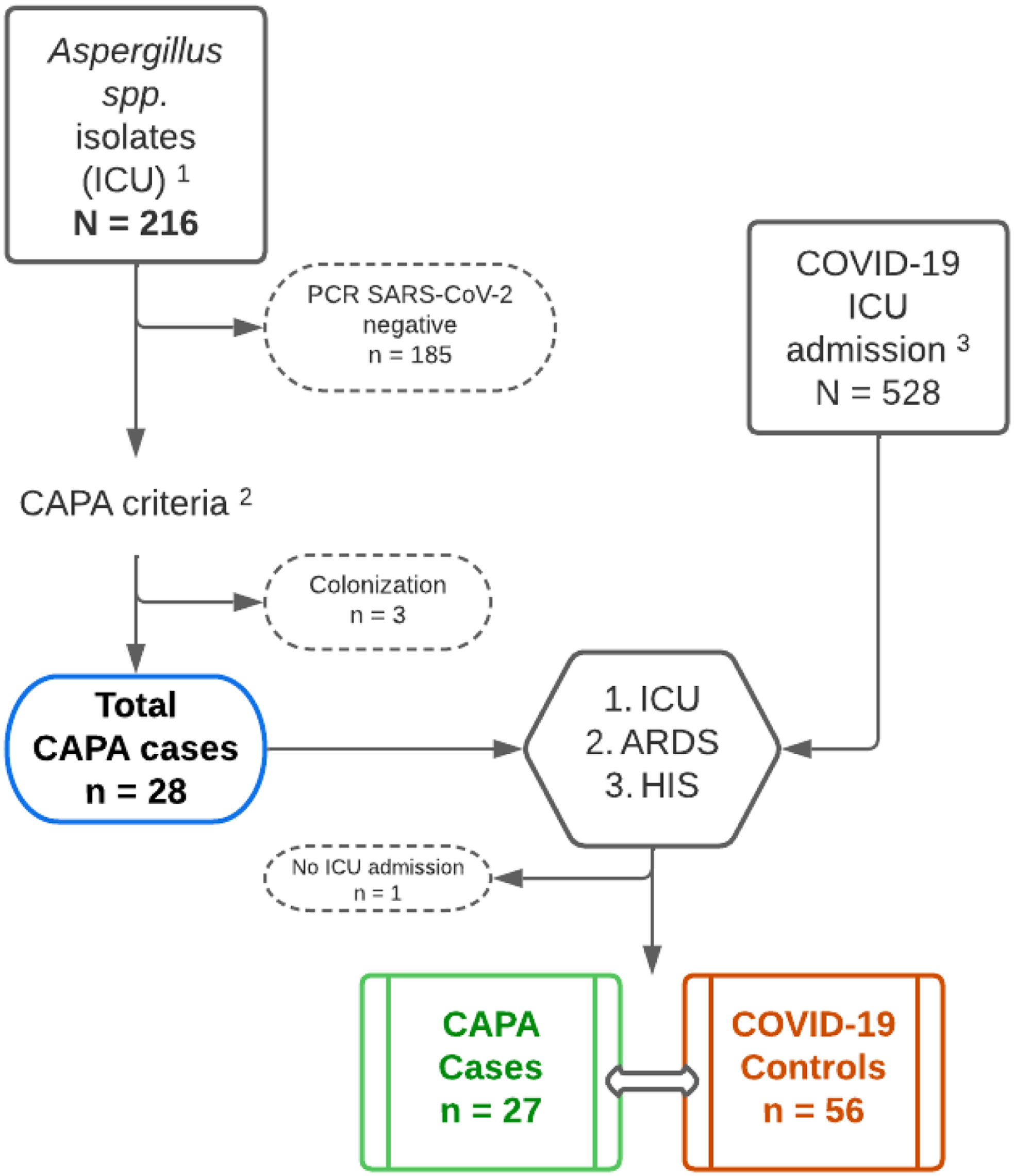
Material and methodsStudy designRetrospective Case–Control Study of Patients diagnosed with COVID-19 pneumonia admitted to the ICU with CAPA criteria between March 2020 and December 2022. Two controls were randomly assigned for each case (Fig. 1). An analysis for CAPA criteria8 was conducted. Severity was defined based on ICU stay, respiratory distress syndrome at admission, and hyperinflammatory syndrome (HIS).27,28
Flowchart of the study population. 1 – Isolation of Aspergillus spp. from respiratory samples provided by the Microbiology Department during the years 2020–2022; 2 – The CAPA criteria used were those proposed by Köhler et al.8; 3 – Patients admitted to the ICU for COVID-19 pneumonia during the years 2020–2022, cases from this cohort were excluded; CAPA: COVID-Associated Pulmonary Aspergillosis; ICU: intensive care unit; ARDS: acute respiratory distress syndrome; HIS: hyperinflammatory syndrome.
The study variables were extracted from the electronic health record. For cases, the analysis included the sample where Aspergillus spp. was isolated, days until the diagnosis, and antifungal treatment. Additionally, the presence of radiological worsening was documented.29 Variables during the course of the infection included whether the patient was directly admitted to the ICU and the days of stay, type of ventilation used and the total days it was required, ECMO and RRT prior to diagnosis, APACHE-II score before admission to the ICU,30 RALE score at admission,31 and treatments received. Nadir of lymphocytes and D-dimer were also registered. Survival variables were recorded.
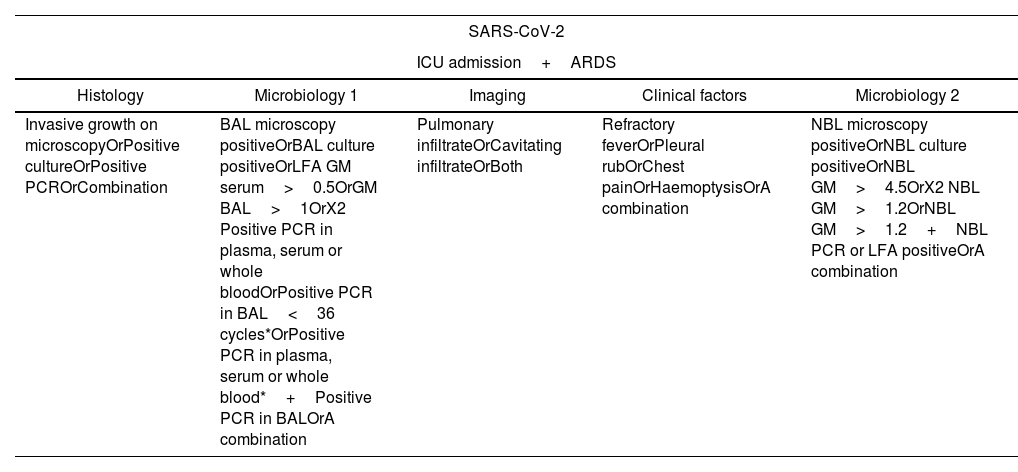
Case definition and microbiological studiesThe cases have been divided into three categories (Appendix 2)8: proven cases, probable cases, possible cases, according to the modification of Köhler et al. criteria. SARS-CoV-2 was detected by means of a commercially available real time PCR (SARS-CoV-2 real time PCR Kit, Vircell, Granada, Spain), testing for the Aspergillus galactomannan in whole blood and BAL fluid was performed on a VirClia autoanalyzer using the Ag VIRCLIA MONOTEST (Vircell, Granada, Spain) with a cut-off for positivity ≥0.5. Standard culture laboratory methods were used for fungi isolation, and isolates were identified using MALDI-TOF technology. Due to the absence of Lateral Flow Assay (LFA) or PCR for Aspergillus spp., GM in plasma samples have been considered in case of positivity with a cut-off ≥0.5. Due to the difficulty in obtaining samples via bronchoscopy during the studied period, we have adapted the criteria defined by Köhler et al. In this adaptation, cases identified through BAL culture have been considered as “probable”. On the other hand, patients identified through cultures of different samples, such as BAS and TAS, have been classified as “possible”. Additionally, it is important to note that, unlike what is established by the original criteria and the usual practice in our center, the determination of the GM antigen has been performed using the VIRCLIA assay instead of Platelia, as we note above.
Statistic analysis and ethical aspectsQuantitative variables were reported as mean and standard deviation or median with interquartile range (IQR), while qualitative variables were expressed as absolute frequencies and percentages. The Saphiro–Wilks test was used to assess the normality of quantitative variables, and as it was significant, the Mann–Whitney U test was employed. For qualitative variables, the Chi-square test or Fisher's Exact test was used. All tests were conducted with a significance level set at p<0.05. A 95% confidence interval was calculated for OR values. The analysis of mortality at 120 days was conducted using the Cox regression model and the Kaplan–Meier method to generate survival curves, with the Log-Rank test (Mantel–Cox) applied. A sensitivity analysis was performed to assess the specificity, negative predictive value, and positive predictive value of different cutoff points for the CAPA score (95% confidence interval). A ROC curve was generated for each cutoff point of the CAPA score. Statistical analyses were conducted using SPSS ® v25.0 (IBM, Armonk, NY, USA) and R-software ® 2023.
The study was approved by the Hospital Ethics Committee of the Hospital Universitario Clínico San Cecilio of Granada (Spain).
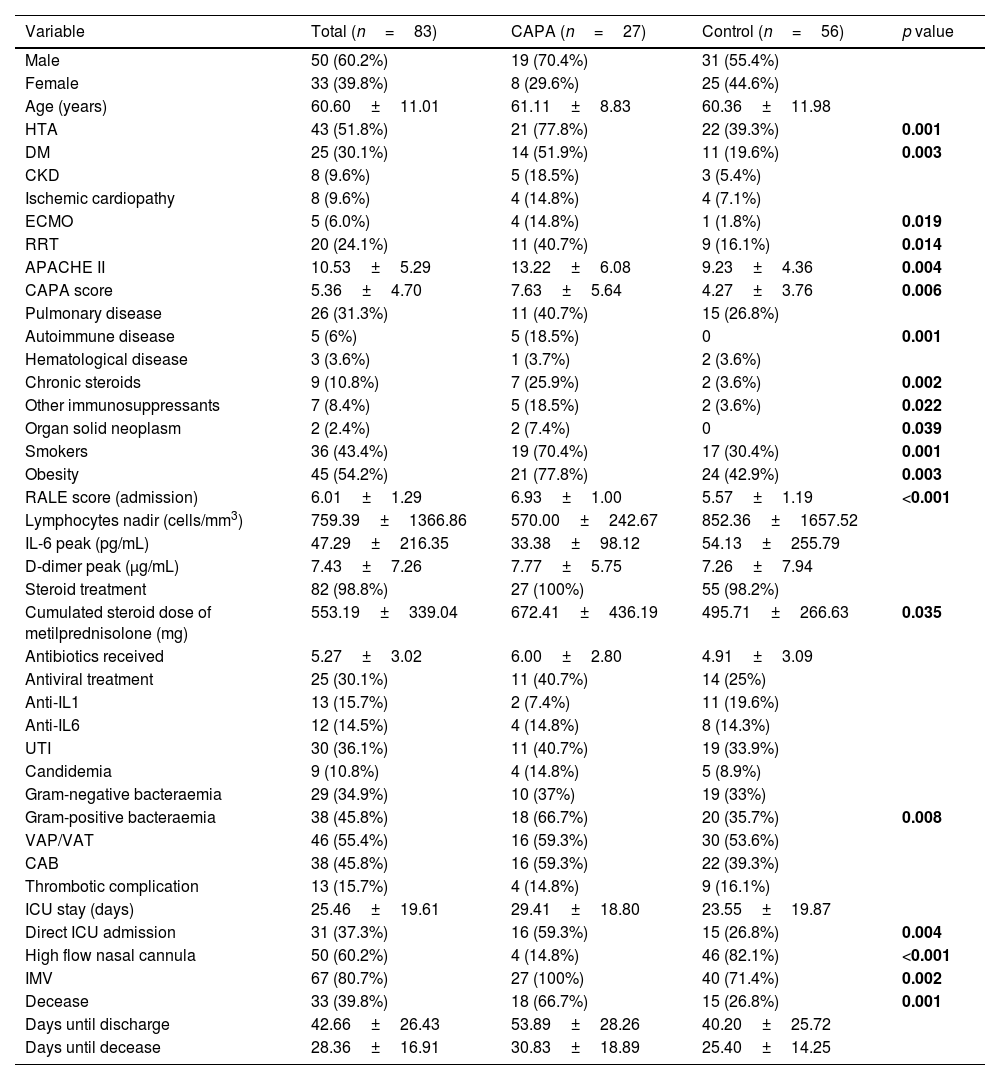
ResultsBaseline characteristics of CAPA cases (Table 1)A total of 27 CAPA cases and 56 controls were recruited. The actual prevalence of CAPA cases was 5.1% if we consider our modified Köhler criteria from 03/2020 to 12/2022. This prevalence would be less than 1% if strict Köhler criteria were applied. The median age of the sample is 61.1 years (IQR 8.8), 70.4% being male. 77.8% had hypertension and obesity, and 40.7% had some form of respiratory disease. 70.4% were smokers, significantly higher among those with CAPA compared to those who did not develop it (70.4% vs. 30.4%; p=0.001). There were significant differences with a higher percentage of patients having hypertension (77.8% vs. 39.3%; p=0.001), diabetes (51.9% vs. 19.6%; p=0.003), smokers, chronic steroid therapy (25.9% vs. 3.6%; p=0.002), and other immunosuppressants (18.5% vs. 3.6%; p=0.022) among CAPA. Autoimmune diseases (18.5% vs. 0%; p=0.001) and solid organ neoplasms (7.4% vs. 0%; p=0.026) was also significantly higher.
Baseline and demographic characteristics of cases and controls.
| Variable | Total (n=83) | CAPA (n=27) | Control (n=56) | p value |
|---|---|---|---|---|
| Male | 50 (60.2%) | 19 (70.4%) | 31 (55.4%) | |
| Female | 33 (39.8%) | 8 (29.6%) | 25 (44.6%) | |
| Age (years) | 60.60±11.01 | 61.11±8.83 | 60.36±11.98 | |
| HTA | 43 (51.8%) | 21 (77.8%) | 22 (39.3%) | 0.001 |
| DM | 25 (30.1%) | 14 (51.9%) | 11 (19.6%) | 0.003 |
| CKD | 8 (9.6%) | 5 (18.5%) | 3 (5.4%) | |
| Ischemic cardiopathy | 8 (9.6%) | 4 (14.8%) | 4 (7.1%) | |
| ECMO | 5 (6.0%) | 4 (14.8%) | 1 (1.8%) | 0.019 |
| RRT | 20 (24.1%) | 11 (40.7%) | 9 (16.1%) | 0.014 |
| APACHE II | 10.53±5.29 | 13.22±6.08 | 9.23±4.36 | 0.004 |
| CAPA score | 5.36±4.70 | 7.63±5.64 | 4.27±3.76 | 0.006 |
| Pulmonary disease | 26 (31.3%) | 11 (40.7%) | 15 (26.8%) | |
| Autoimmune disease | 5 (6%) | 5 (18.5%) | 0 | 0.001 |
| Hematological disease | 3 (3.6%) | 1 (3.7%) | 2 (3.6%) | |
| Chronic steroids | 9 (10.8%) | 7 (25.9%) | 2 (3.6%) | 0.002 |
| Other immunosuppressants | 7 (8.4%) | 5 (18.5%) | 2 (3.6%) | 0.022 |
| Organ solid neoplasm | 2 (2.4%) | 2 (7.4%) | 0 | 0.039 |
| Smokers | 36 (43.4%) | 19 (70.4%) | 17 (30.4%) | 0.001 |
| Obesity | 45 (54.2%) | 21 (77.8%) | 24 (42.9%) | 0.003 |
| RALE score (admission) | 6.01±1.29 | 6.93±1.00 | 5.57±1.19 | <0.001 |
| Lymphocytes nadir (cells/mm3) | 759.39±1366.86 | 570.00±242.67 | 852.36±1657.52 | |
| IL-6 peak (pg/mL) | 47.29±216.35 | 33.38±98.12 | 54.13±255.79 | |
| D-dimer peak (μg/mL) | 7.43±7.26 | 7.77±5.75 | 7.26±7.94 | |
| Steroid treatment | 82 (98.8%) | 27 (100%) | 55 (98.2%) | |
| Cumulated steroid dose of metilprednisolone (mg) | 553.19±339.04 | 672.41±436.19 | 495.71±266.63 | 0.035 |
| Antibiotics received | 5.27±3.02 | 6.00±2.80 | 4.91±3.09 | |
| Antiviral treatment | 25 (30.1%) | 11 (40.7%) | 14 (25%) | |
| Anti-IL1 | 13 (15.7%) | 2 (7.4%) | 11 (19.6%) | |
| Anti-IL6 | 12 (14.5%) | 4 (14.8%) | 8 (14.3%) | |
| UTI | 30 (36.1%) | 11 (40.7%) | 19 (33.9%) | |
| Candidemia | 9 (10.8%) | 4 (14.8%) | 5 (8.9%) | |
| Gram-negative bacteraemia | 29 (34.9%) | 10 (37%) | 19 (33%) | |
| Gram-positive bacteraemia | 38 (45.8%) | 18 (66.7%) | 20 (35.7%) | 0.008 |
| VAP/VAT | 46 (55.4%) | 16 (59.3%) | 30 (53.6%) | |
| CAB | 38 (45.8%) | 16 (59.3%) | 22 (39.3%) | |
| Thrombotic complication | 13 (15.7%) | 4 (14.8%) | 9 (16.1%) | |
| ICU stay (days) | 25.46±19.61 | 29.41±18.80 | 23.55±19.87 | |
| Direct ICU admission | 31 (37.3%) | 16 (59.3%) | 15 (26.8%) | 0.004 |
| High flow nasal cannula | 50 (60.2%) | 4 (14.8%) | 46 (82.1%) | <0.001 |
| IMV | 67 (80.7%) | 27 (100%) | 40 (71.4%) | 0.002 |
| Decease | 33 (39.8%) | 18 (66.7%) | 15 (26.8%) | 0.001 |
| Days until discharge | 42.66±26.43 | 53.89±28.26 | 40.20±25.72 | |
| Days until decease | 28.36±16.91 | 30.83±18.89 | 25.40±14.25 |
CAPA: COVID-Associated Pulmonary Aspergillosis; HTA: hypertension; DM: diabetes mellitus; CKD: chronic kidney disease; ECMO: extracorporeal membrane oxygenation; RRT: renal replacement therapy; UTI: urinary tract infection; VAP/VAT: ventilation-associated pneumonia/ventilation-associated tracheobronchitis; CAB: catheter-associated bacteremia; ICU: intensive care unit; IMV: invasive mechanical ventilation.
CAPA cases presented with higher RALE score at hospital admission (6.9±1.00 vs 5.6±1.2; p<0.001), higher APACHE-II scores (13.2±6.1 vs 9.2±4.4; p=0.004), and they were more likely to be directly admitted to the ICU (59.3% vs 26.8%; p=0.004). All cases had invasive ventilatory support before the diagnosis, with ECMO in 14.8% (14.8% vs 1.8%; p=0.019), and they received RRT more frequently (40.7% vs 16.1%; p=0.014). Significant differences were noted in the cumulative dose of corticosteroids before the diagnosis, which was higher in CAPA (672.4mg±436.2 vs 495.7±266.6mg; p=0.035). Finally, patients with CAPA had higher mortality at 120 days rates than those who did not develop it (66.7% vs 26.8%; p=0.001).
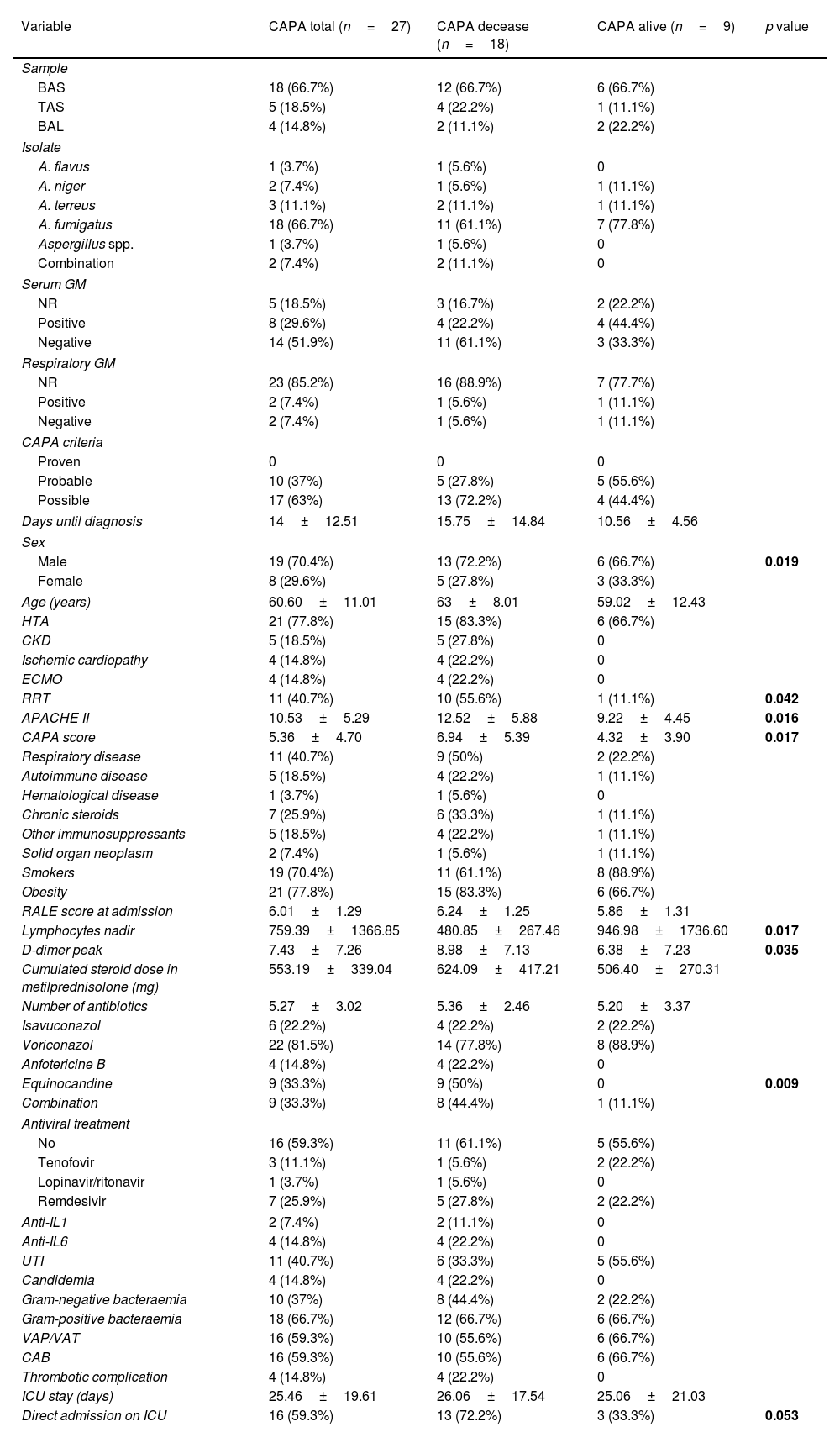
Analysis of CAPA cases (Tables 2 and 3)A 66.7% were diagnosed based on microbiological isolation in a bronchial aspirate, and 14.8% in bronchoalveolar lavage. 66.7% of the isolates were Aspergillus fumigatus. Galactomannan (GM) determinations in serum were negative in 51.9%. GM determinations in respiratory samples were performed in 14.8% of patients, being positive in 50% patients tested. 63% of CAPA were classified as “possible” CAPA, and 37% as “probable” CAPA. The median time to CAPA diagnosis was 14 days from hospital admission. The percentage of males (72.2% vs 66.7%; p=0.019), type 2 diabetics (72.2% vs 11.1%; p=0.004), RRT (55.6% vs 11.1%; p=0.042), APACHE II (12.5 vs 9.2; p=0.016), CAPA score (6.9 vs 4.3; p=0.017), nadir lymphocytes (481±1366.9 vs 947±1736.6 lymphocytes per mm3; p=0.017), and peak D-dimer (9±7.1 vs 6.4±7.2mcg/mL; p=0.035) were higher among deceased CAPA cases.
Characteristics of CAPA cases comparing deceased and survivors.
| Variable | CAPA total (n=27) | CAPA decease (n=18) | CAPA alive (n=9) | p value |
|---|---|---|---|---|
| Sample | ||||
| BAS | 18 (66.7%) | 12 (66.7%) | 6 (66.7%) | |
| TAS | 5 (18.5%) | 4 (22.2%) | 1 (11.1%) | |
| BAL | 4 (14.8%) | 2 (11.1%) | 2 (22.2%) | |
| Isolate | ||||
| A. flavus | 1 (3.7%) | 1 (5.6%) | 0 | |
| A. niger | 2 (7.4%) | 1 (5.6%) | 1 (11.1%) | |
| A. terreus | 3 (11.1%) | 2 (11.1%) | 1 (11.1%) | |
| A. fumigatus | 18 (66.7%) | 11 (61.1%) | 7 (77.8%) | |
| Aspergillus spp. | 1 (3.7%) | 1 (5.6%) | 0 | |
| Combination | 2 (7.4%) | 2 (11.1%) | 0 | |
| Serum GM | ||||
| NR | 5 (18.5%) | 3 (16.7%) | 2 (22.2%) | |
| Positive | 8 (29.6%) | 4 (22.2%) | 4 (44.4%) | |
| Negative | 14 (51.9%) | 11 (61.1%) | 3 (33.3%) | |
| Respiratory GM | ||||
| NR | 23 (85.2%) | 16 (88.9%) | 7 (77.7%) | |
| Positive | 2 (7.4%) | 1 (5.6%) | 1 (11.1%) | |
| Negative | 2 (7.4%) | 1 (5.6%) | 1 (11.1%) | |
| CAPA criteria | ||||
| Proven | 0 | 0 | 0 | |
| Probable | 10 (37%) | 5 (27.8%) | 5 (55.6%) | |
| Possible | 17 (63%) | 13 (72.2%) | 4 (44.4%) | |
| Days until diagnosis | 14±12.51 | 15.75±14.84 | 10.56±4.56 | |
| Sex | ||||
| Male | 19 (70.4%) | 13 (72.2%) | 6 (66.7%) | 0.019 |
| Female | 8 (29.6%) | 5 (27.8%) | 3 (33.3%) | |
| Age (years) | 60.60±11.01 | 63±8.01 | 59.02±12.43 | |
| HTA | 21 (77.8%) | 15 (83.3%) | 6 (66.7%) | |
| CKD | 5 (18.5%) | 5 (27.8%) | 0 | |
| Ischemic cardiopathy | 4 (14.8%) | 4 (22.2%) | 0 | |
| ECMO | 4 (14.8%) | 4 (22.2%) | 0 | |
| RRT | 11 (40.7%) | 10 (55.6%) | 1 (11.1%) | 0.042 |
| APACHE II | 10.53±5.29 | 12.52±5.88 | 9.22±4.45 | 0.016 |
| CAPA score | 5.36±4.70 | 6.94±5.39 | 4.32±3.90 | 0.017 |
| Respiratory disease | 11 (40.7%) | 9 (50%) | 2 (22.2%) | |
| Autoimmune disease | 5 (18.5%) | 4 (22.2%) | 1 (11.1%) | |
| Hematological disease | 1 (3.7%) | 1 (5.6%) | 0 | |
| Chronic steroids | 7 (25.9%) | 6 (33.3%) | 1 (11.1%) | |
| Other immunosuppressants | 5 (18.5%) | 4 (22.2%) | 1 (11.1%) | |
| Solid organ neoplasm | 2 (7.4%) | 1 (5.6%) | 1 (11.1%) | |
| Smokers | 19 (70.4%) | 11 (61.1%) | 8 (88.9%) | |
| Obesity | 21 (77.8%) | 15 (83.3%) | 6 (66.7%) | |
| RALE score at admission | 6.01±1.29 | 6.24±1.25 | 5.86±1.31 | |
| Lymphocytes nadir | 759.39±1366.85 | 480.85±267.46 | 946.98±1736.60 | 0.017 |
| D-dimer peak | 7.43±7.26 | 8.98±7.13 | 6.38±7.23 | 0.035 |
| Cumulated steroid dose in metilprednisolone (mg) | 553.19±339.04 | 624.09±417.21 | 506.40±270.31 | |
| Number of antibiotics | 5.27±3.02 | 5.36±2.46 | 5.20±3.37 | |
| Isavuconazol | 6 (22.2%) | 4 (22.2%) | 2 (22.2%) | |
| Voriconazol | 22 (81.5%) | 14 (77.8%) | 8 (88.9%) | |
| Anfotericine B | 4 (14.8%) | 4 (22.2%) | 0 | |
| Equinocandine | 9 (33.3%) | 9 (50%) | 0 | 0.009 |
| Combination | 9 (33.3%) | 8 (44.4%) | 1 (11.1%) | |
| Antiviral treatment | ||||
| No | 16 (59.3%) | 11 (61.1%) | 5 (55.6%) | |
| Tenofovir | 3 (11.1%) | 1 (5.6%) | 2 (22.2%) | |
| Lopinavir/ritonavir | 1 (3.7%) | 1 (5.6%) | 0 | |
| Remdesivir | 7 (25.9%) | 5 (27.8%) | 2 (22.2%) | |
| Anti-IL1 | 2 (7.4%) | 2 (11.1%) | 0 | |
| Anti-IL6 | 4 (14.8%) | 4 (22.2%) | 0 | |
| UTI | 11 (40.7%) | 6 (33.3%) | 5 (55.6%) | |
| Candidemia | 4 (14.8%) | 4 (22.2%) | 0 | |
| Gram-negative bacteraemia | 10 (37%) | 8 (44.4%) | 2 (22.2%) | |
| Gram-positive bacteraemia | 18 (66.7%) | 12 (66.7%) | 6 (66.7%) | |
| VAP/VAT | 16 (59.3%) | 10 (55.6%) | 6 (66.7%) | |
| CAB | 16 (59.3%) | 10 (55.6%) | 6 (66.7%) | |
| Thrombotic complication | 4 (14.8%) | 4 (22.2%) | 0 | |
| ICU stay (days) | 25.46±19.61 | 26.06±17.54 | 25.06±21.03 | |
| Direct admission on ICU | 16 (59.3%) | 13 (72.2%) | 3 (33.3%) | 0.053 |
BAS: bronchial aspirate; TAS: tracheal aspirate; BAL: bronchoalveolar lavage; GM; galactomannan; NR: non-requested; CAPA: COVID-Associated Pulmonary Aspergillosis; HTA: hypertension; DM: diabetes mellitus; CKD: chronic kidney disease; ECMO: extracorporeal membrane oxygenation; RRT: renal replacement therapy; UTI: urinary tract infection; VAP/VAT: ventilation-associated pneumonia/ventilation-associated tracheobronchitis; CAB: catheter-associated bacteremia; ICU: intensive care unit; IMV: invasive mechanical ventilation.
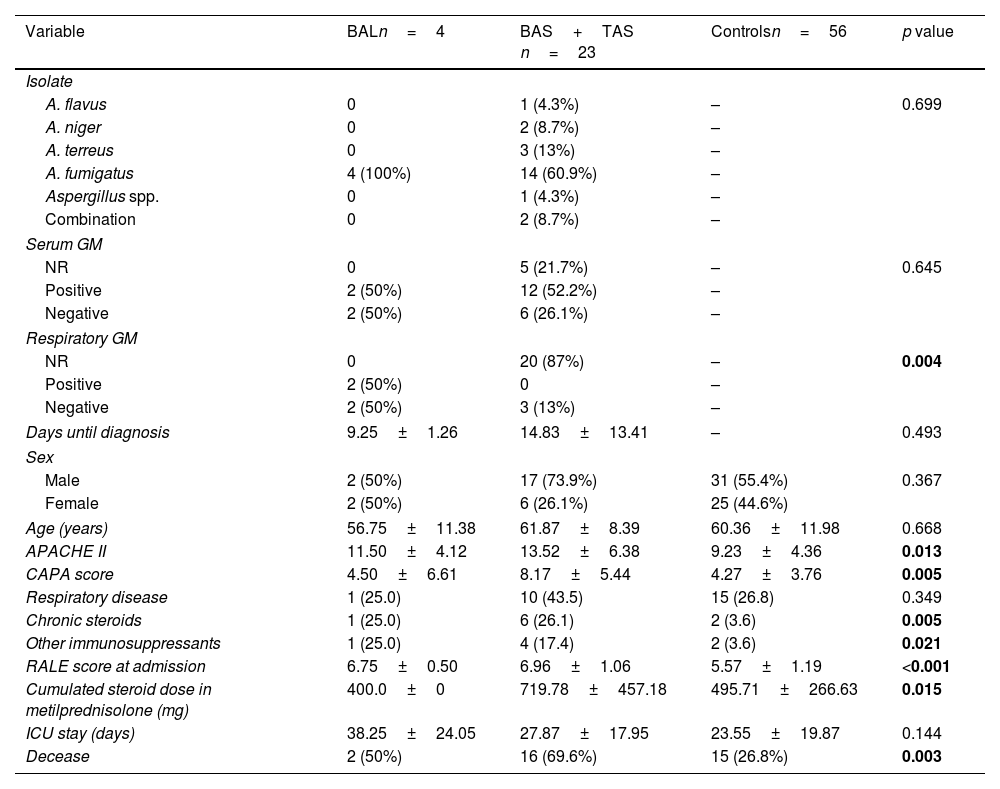
Comparative analysis between CAPA diagnosed through BAL and other samples.
| Variable | BALn=4 | BAS+TAS n=23 | Controlsn=56 | p value |
|---|---|---|---|---|
| Isolate | ||||
| A. flavus | 0 | 1 (4.3%) | – | 0.699 |
| A. niger | 0 | 2 (8.7%) | – | |
| A. terreus | 0 | 3 (13%) | – | |
| A. fumigatus | 4 (100%) | 14 (60.9%) | – | |
| Aspergillus spp. | 0 | 1 (4.3%) | – | |
| Combination | 0 | 2 (8.7%) | – | |
| Serum GM | ||||
| NR | 0 | 5 (21.7%) | – | 0.645 |
| Positive | 2 (50%) | 12 (52.2%) | – | |
| Negative | 2 (50%) | 6 (26.1%) | – | |
| Respiratory GM | ||||
| NR | 0 | 20 (87%) | – | 0.004 |
| Positive | 2 (50%) | 0 | – | |
| Negative | 2 (50%) | 3 (13%) | – | |
| Days until diagnosis | 9.25±1.26 | 14.83±13.41 | – | 0.493 |
| Sex | ||||
| Male | 2 (50%) | 17 (73.9%) | 31 (55.4%) | 0.367 |
| Female | 2 (50%) | 6 (26.1%) | 25 (44.6%) | |
| Age (years) | 56.75±11.38 | 61.87±8.39 | 60.36±11.98 | 0.668 |
| APACHE II | 11.50±4.12 | 13.52±6.38 | 9.23±4.36 | 0.013 |
| CAPA score | 4.50±6.61 | 8.17±5.44 | 4.27±3.76 | 0.005 |
| Respiratory disease | 1 (25.0) | 10 (43.5) | 15 (26.8) | 0.349 |
| Chronic steroids | 1 (25.0) | 6 (26.1) | 2 (3.6) | 0.005 |
| Other immunosuppressants | 1 (25.0) | 4 (17.4) | 2 (3.6) | 0.021 |
| RALE score at admission | 6.75±0.50 | 6.96±1.06 | 5.57±1.19 | <0.001 |
| Cumulated steroid dose in metilprednisolone (mg) | 400.0±0 | 719.78±457.18 | 495.71±266.63 | 0.015 |
| ICU stay (days) | 38.25±24.05 | 27.87±17.95 | 23.55±19.87 | 0.144 |
| Decease | 2 (50%) | 16 (69.6%) | 15 (26.8%) | 0.003 |
BAS: bronchial aspirate; TAS: tracheal aspirate; BAL: bronchoalveolar lavage; GM; galactomannan; NR: non-requested; CAPA: COVID-Associated Pulmonary Aspergillosis; HTA: ICU: intensive care unit.
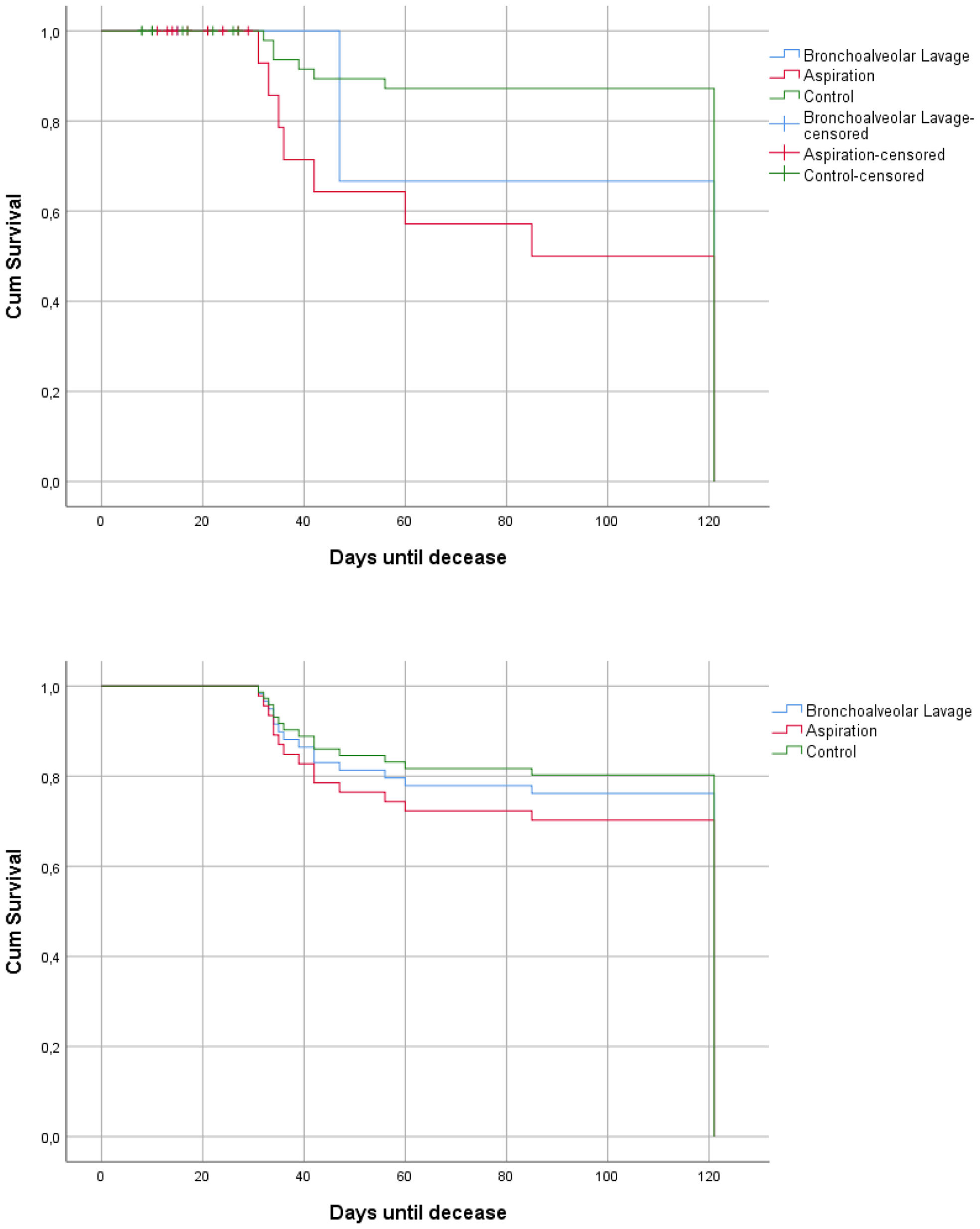
A comparative analysis was conducted between patients diagnosed through BAL and those diagnosed using other samples (Table 3). The serum GM positivity rate was 52.2% in patients diagnosed with samples other than BAL, and 50% in those diagnosed through BAL. The median time to diagnosis was 14.8 days for patients diagnosed via BAS or TAS, compared to 9.3 days for BAL patients, although no significant differences were found. The APACHE II score was 13.5 points in patients not diagnosed by BAL and 11.5 points in those diagnosed by BAL (p=0.013). Regarding the CAPA score, patients diagnosed via BAS and TAS had an average of 8.2 points, while those diagnosed through BAL had an average of 4.5 points (p=0.005). The cumulative dose of steroids was 400mg in BAL patients and 719.8mg in those diagnosed with BAS and TAS (p=0.015). Finally, the mortality rate at 120 days in the BAL group was 50%, compared to 69.6% in the BAS and TAS group (p=0.03).
Risk factors associated with the development of CAPAFor hypertension, type 2 diabetes mellitus, smoking, and obesity, an OR of 5.41 (95% CI [1.998–14.861], p=0.001), 4.41 (95% CI [1.617–12.002], p=0.003), 5.45 (95% CI [1.998–14.861], p=0.001), and 4.67 (95% CI [1.632–13.341], p=0.003) was obtained, respectively. Regarding autoimmune disease, the OR was 1.23 (95% CI [1.025–1.469], p=0.001). Chronic corticosteroid therapy was associated with an OR of 9.45 (95% CI [1.809–49.358], p=0.005), as well as the use of other immunosuppressants (OR 6.14; 95% CI [1.106–34.034], p=0.034).
CAPA score greater than or equal to 5 points, RALE score greater than or equal to 5 points, and APACHE II score at admission to the ICU greater than 10 points yielded an OR of 3.33 (95% CI [1.269–8.757], p=0.013), 6.36 (95% CI [0.776–52.071], p=0.053), and 3.17 (95% CI [1.187–8.446], p=0.019), respectively. Those who received RRT, ECMO, and a cumulative dose of corticosteroids>800mg of methylprednisolone obtained an OR of 3.59 (95% CI [1.259–10.237], p=0.014), 9.57 (95% CI [1.014–90.273], p=0.037), and 3.57 (95% CI [1.014–12.570], p=0.039), respectively. Direct admission to the ICU and the use of IMV also yielded a significant OR of 3.98 (95% CI [1.509–10.478], p=0.004) and OR 1.68 (95% CI [1.376–2.039], p=0.002), respectively.
Finally, the fact of developing CAPA was at a higher risk of mortality at 120 days (OR 5.47; 95% CI [2.021–14.786], p=0.001).
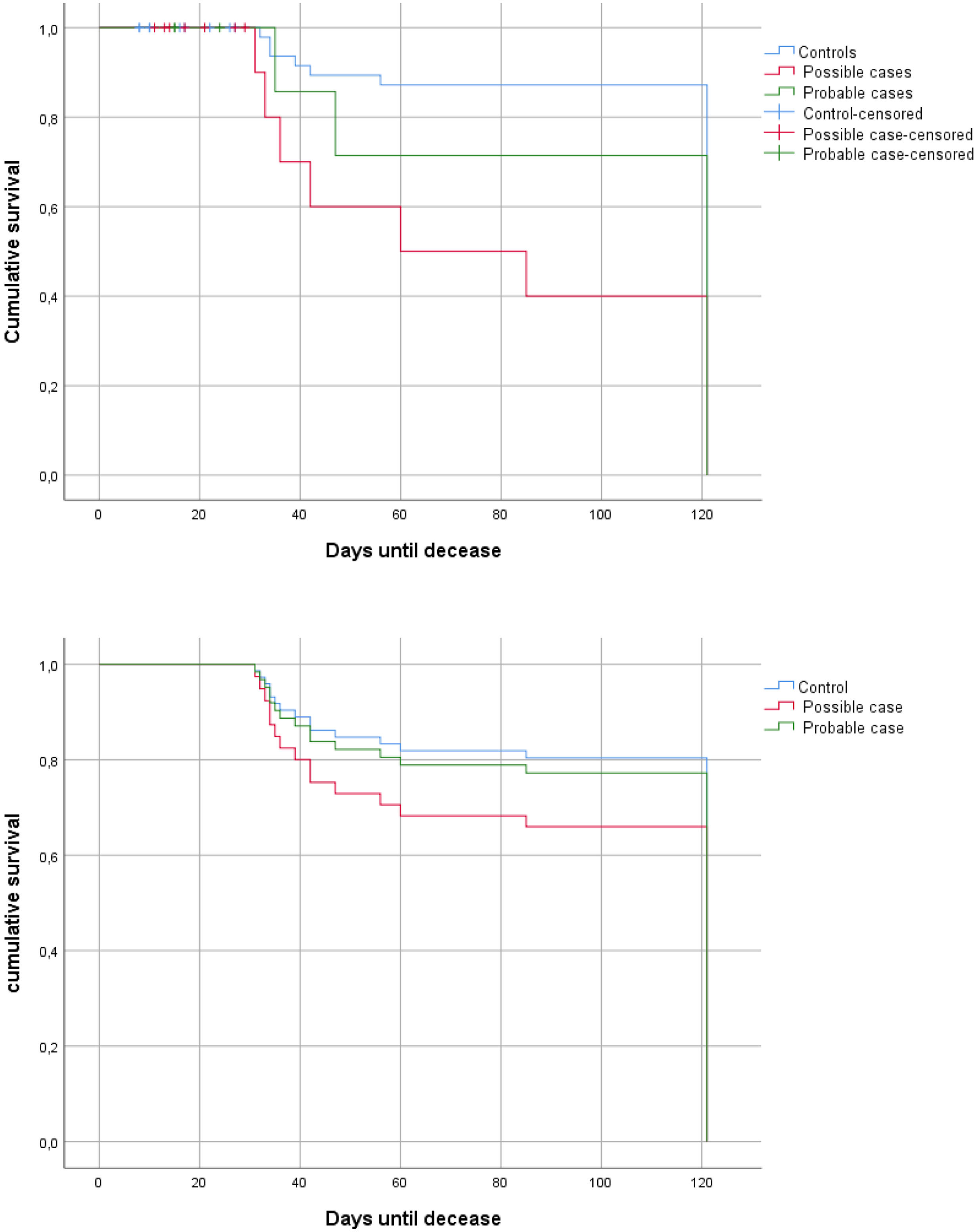
Mortality analysisA 66.7% of mortality at 120 days were identified for CAPA patients. A mortality analysis at 120 days was conducted using the Kaplan–Meier method. According to the Log-Rank analysis, there were significant differences between patients identified based on the risk of developing CAPA (“probable” and “possible”) and controls (Fig. 2a). However, when the Cox regression model is applied, it is not statistically significant (Fig. 2b) (HR1=0.84, 95% CI [0.381; 1.867], p=0.675; HR2=1.61, 95% CI [0.612; 4.235], p=0.334).
(a) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) using Log-Rank; (b) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) applying Cox regression model. Y axis: cumulative survival; X axis: days until decease.
Additionally, a differentiated mortality analysis at 120 days was conducted based on the type of sample used for diagnosis, revealing significant differences between patients diagnosed via BAL compared to BAS and TAS. However, as in the previous analysis, when performing the Cox regression, these differences were not statistically significant (Fig. 3a and b).
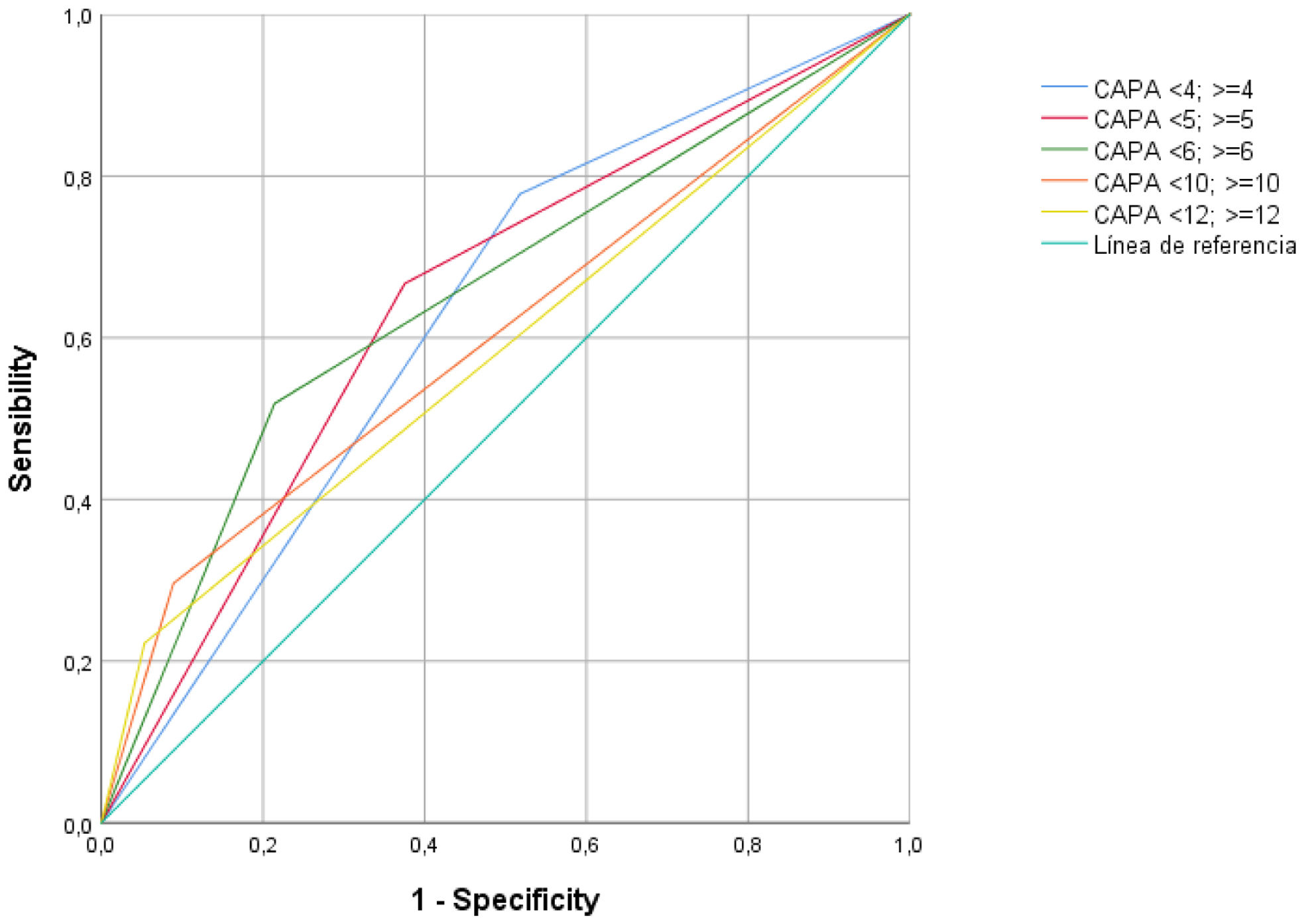
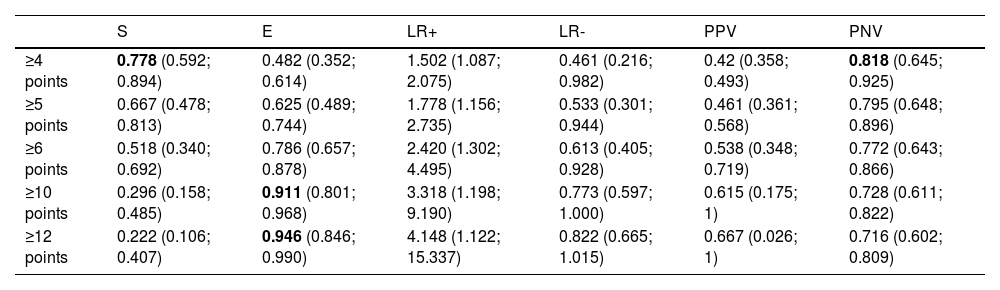
CAPA score26A table of sensitivity, specificity, and predictive values was created (Table 4). Sensitivity values of 0.78 (95% CI [0.592; 0.894]) were obtained for CAPA score values greater than or equal to 4 points, corresponding to a negative predictive value (NPV) of 82% (NPV=0.818; 95% CI [0.645; 0.925]). To achieve specificities of 91%, a score of 10 points or higher is required (E=0.911; 95% CI [0.801; 0.968]). The ROC curves for each cutoff point are shown in Fig. 4.
Sensitivity and specificity analysis of the CAPA score in our cohort.
| S | E | LR+ | LR- | PPV | PNV | |
|---|---|---|---|---|---|---|
| ≥4 points | 0.778 (0.592; 0.894) | 0.482 (0.352; 0.614) | 1.502 (1.087; 2.075) | 0.461 (0.216; 0.982) | 0.42 (0.358; 0.493) | 0.818 (0.645; 0.925) |
| ≥5 points | 0.667 (0.478; 0.813) | 0.625 (0.489; 0.744) | 1.778 (1.156; 2.735) | 0.533 (0.301; 0.944) | 0.461 (0.361; 0.568) | 0.795 (0.648; 0.896) |
| ≥6 points | 0.518 (0.340; 0.692) | 0.786 (0.657; 0.878) | 2.420 (1.302; 4.495) | 0.613 (0.405; 0.928) | 0.538 (0.348; 0.719) | 0.772 (0.643; 0.866) |
| ≥10 points | 0.296 (0.158; 0.485) | 0.911 (0.801; 0.968) | 3.318 (1.198; 9.190) | 0.773 (0.597; 1.000) | 0.615 (0.175; 1) | 0.728 (0.611; 0.822) |
| ≥12 points | 0.222 (0.106; 0.407) | 0.946 (0.846; 0.990) | 4.148 (1.122; 15.337) | 0.822 (0.665; 1.015) | 0.667 (0.026; 1) | 0.716 (0.602; 0.809) |
S: sensitivity; E: specificity; LR: likelihood ratio; PPV: predictive positive value; PNV: predictive negative value.
ROC curve for all cutoff points obtained from the CAPA score.26 Reference line in cyan color.
A total of 27 cases of CAPA have been recruited between the years 2020 and 2022. The prevalence was 5.1%, similar to published studies, reaching up to 15% of patients admitted to the ICU for severe COVID-19 pneumonia.6,11,18,22,23 This variation is mainly attributed to the broad variability in criteria used to classify patients as CAPA. Despite employing one of the classifications8 that exhibits high sensitivity in detecting CAPA, the prevalence was lower compared to similar studies. This discrepancy may stem from the low number of BALs performed.6,8,18,22 The classification as “possible” CAPA was the predominant (63%). Thanks to the adaptation of Köhler et al.’s criteria, it has been possible to diagnose patients who, due to the severity of their condition, have not been candidates for BAL. The prevalence of these cases, ranging from <1% to 5.1%, is similar to that reported in the existing literature.
The median time to diagnosis from admission was 14 days. This temporal aspect is greater compared to previously published data ranging between 7 and 8 days,6,17,23 suggesting a potential diagnostic delay in our population. Moreover, the likely delay in performing culture tests, galactomannan in respiratory and plasma samples, due to low diagnostic suspicion, combined with the absence of rapid diagnostic tests such as LFA, may have caused a diagnostic delay of up to 3–4 days with the associated prognostic consequences. Although not statistically significant, the time to diagnosis in the CAPA patients who survived was shorter than in those who did not (15.75±14.84 and 10.56±4.56). Ultimately, the “possible” category, despite the low specificity of isolation in respiratory samples others than BAL, allows for early treatment in patients where BAL cannot be promptly performed or is contraindicated.32,33 Additionally, patients considered colonizers in other series have been shown to have a worse prognosis than non-colonized individuals. This is well-established in other settings outside of SARS-CoV-2, such as COPD. Fortún et al.33 emphasizes the high mortality among colonized patients compared to cases. The identification of patients colonized by Aspergillus spp. should alert us to the need for using all available diagnostic tools to promptly diagnose IPA, and the high mortality demonstrated in colonized likely arises from cases of aspergillosis that we are unable to diagnose.32–34 We also emphasize this aspect in Table 3 and Fig. 3, where we demonstrate that patients diagnosed through samples like BAS or TAS have a worse prognosis because they are usually diagnosed later or are more severely ill patients who are not candidates for BAL. The risk factors identified in patients diagnosed through BAL are even more striking in this subgroup, as seen with the APACHE II score, the CAPA score, and the cumulative dose of steroids. Another conclusion we can draw from the data is that isolating Aspergillus in samples other than BAL leads to a higher likelihood of considering colonization, which delays the response time to a possible CAPA diagnosis. This is reflected in our study by the significantly longer time to diagnosis in the subgroup of patients diagnosed via BAS or TAS (approximately 14 vs. 8 days). This fact is also reflected in the significant higher rate of mortality in BAS and TAS patient compared to BAL or controls.
Most CAPA patients were diagnosed based on bronchial aspirate (BAS), with A. fumigatus being the most prevalent species (66.7%).6 BAS and TAS samples are less specific than BAL, potentially leading to an overestimation of CAPA prevalence. However, there is good correlation between BAS and BAL samples.1 Plasmatic GM was negative in 51.9% of cases, supporting the idea that GM is a less sensitive but specific marker for invasive infection. It has been demonstrated that GM in BAL is more sensitive than in serum for CAPA.6,34 The sensitivity of fungal markers in plasma for CAPA has a limited role. This not only affects diagnostic markers but also imaging tests, making it very difficult to distinguish findings of COVID-19 from those of aspergillosis.35,36 Further studies are needed to determine more precisely the role of this marker in non-invasive samples. Additionally, encouraging the use of rapid diagnostic tools like the LFA, which correlates well with GM values in BAL or plasma, is essential.33
Baseline characteristics reveal a majority of males aged 60 years or older as the most common profile for CAPA cases, consistent with previous findings.7,11 A significant difference was demonstrated between CAPA cases and controls regarding the presence of cardiovascular risk factors, autoimmune diseases, and solid organ neoplasms. Other immunosuppressants were associated with up to a 6-fold increase in the development of CAPA. There is variability compared to published data, with a higher prevalence of type 2 diabetes mellitus and obesity without statistical significance.7 Chronic corticosteroids have been previously demonstrated as a well-established risk factor for the development of CAPA and IAPA.6,11,12 An important aspect to highlight is the significant difference in the presence of autoimmune diseases, likely a result of their chronic immunosuppressive treatment.
Significant differences regarding a history of respiratory diseases have not been demonstrated, despite various published studies confirming it as a significant risk factor.6,7 Concerning classic host factors associated with invasive fungal infections, the prevalence in our center was low. Therefore, we believe that these population may be underestimated in our study.10,24,37 This underscores the importance of identifying CAPA even in patients who do not exhibit the classically identified risk factors for developing IPA.
We highlight the difference in the increased use of ECMO and RRT in patients with CAPA prior to their diagnosis. Either the use of ECMO and/or RRT are probably precipitating factors for the development of CAPA, or the need for this type of support is an additional severity factor in the context of developing CAPA. The need for ECMO and RRT has been identified as a risk factor for IPA17,37; A recent study has published the association between COVID-19 and an increased risk of invasive fungal infection in patients on ECMO as an independent risk factor which would support our findings.7,17,38 The risk assessment scales showed a higher risk among those with a high punctuation on APACHE-II, CAPA, and RALE score. Different risk assessment tools have been used in our study in addition to APACHE-II, such as the SOFA score,7 which has been shown to be also a significant risk factor.
“CAPA score” is introduced as the first score developed for the early identification of this complication in patients with severe COVID-19.26 Although it has proven to be a significant risk factor in our population, the subsequent analysis (Table 4) indicates that a score of 10 or more are needed to achieve specificities greater than 90%. For a score of 4 or more, sensitivity does not reach 80%, although it does surpass a negative predictive value of 81%. This is probably due to the inclusion of risk factors in its development that have not been identified in our population. Due to the small sample size in our study, it is not possible to externally validate this score; however, its utility in our population would be limited.
One of the most interesting aspects of the study is the identification of direct admission to the ICU from the Emergency Department as a risk factor. This has already been suggested in previous publications.7,11,17 However, this would be the first study in which direct admission to the ICU from the Emergency Department without prior hospital ward admission is a risk factor “per se” for the development of CAPA.
The role of corticosteroids has been widely discussed in the context of COVID-19 infection as a risk factor for developing CAPA, with contradictory results.6,7,18,22 Since most patients receive corticosteroids in the context of COVID-19 infection, it is possible that it may be a poor predictor of CAPA. However, the cumulative dose of corticosteroids could be a key factor for those at special risk. In our case, a cumulative dose equivalent to 800mg or more of methylprednisolone has been significantly associated with an increased risk of developing CAPA. This is one of the key aspects of our study since, until now, there are no studies regarding the dose at which the risk of CAPA increases, as the use of steroids in this population is widespread. Immunomodulators have not shown an increased risk of CAPA,11 even though some studies have demonstrated that the use of tocilizumab has been identified as a risk factor.18,22,26 This is likely related to the fact that the prescription of tocilizumab during the pandemic has been variable across different periods.
The presence of CAPA was associated with an excess mortality of up to 5 times, with a mortality rate among cases of 66.7%. Nevertheless, when applying the Cox regression model, no significant differences were observed, although they were clinically significant. This is related to the limited sample size of the study. A similar situation occurs in the analysis of mortality at 120 days between “possible” and “probable” CAPA cases. In the multicenter MYCOVID study conducted across various French centers, similar diagnostic criteria, including non-BAL samples such as BAS and TAS, and mortality rates were identified (61.8%).18
Our study has limitations inherent to its design, and the low number of cases, which is related to the characteristics of our population. This also contributes to limitations in identifying statistical significance in some of the analyzed variables and the mortality analysis. Another noteworthy aspect is the low percentage of BAL samples which may also lead to an overdiagnosis of CAPA in the analyzed population, and the low percentage of GM determinations in respiratory samples is likely due to the heterogeneity in the management of these patients during these first months of the pandemic. This last aspect prevents Kohler's criteria from being strictly applied and has led to their adaptation to the usual clinical practice in our hospital. Therefore, there are inherent biases in this aspect. Another point is the fact that the consensus and criteria of recent years are based on Platelia (EIA), there are studies that support the use and compare VirClia (CLIA), although it is true that the population of this study were hematological patients or those who received hematopoietic progenitor transplants. The sensitivity values in this study are even higher for CLIA compared to EIA in BAL. However, it is true, more studies outside this population (e.g., CAPA) are needed, and they are not validated in all non-BAL samples.39
In summary, we conclude that CAPA is a complication with a high mortality at 120 days rate (67%) in patients with severe pneumonia due to SARS-CoV-2, largely determined by the stay in the ICU. There are host-specific risk factors in these patients, such as those related to cardiovascular diseases, autoimmune diseases, and chronic immunosuppressive treatment, which differ from the classic factors. The high dose of corticosteroids emerges as a key aspect in assessing the risk of CAPA. Early ICU admission, ECMO and RRT emerges as well stablished risk factors which were not clearly suggested previously. Although CAPA score is a useful tool, larger studies are needed to complete its validation. Non-invasive diagnostic tools developed in more heterogeneous populations are needed to identify which patients more accurately would benefit from prophylactic treatment or close monitoring of CAPA development to reduce the excess mortality associated with this feared complication. We believe that the flexibility of the criteria proposed in the discussed consensus is crucial when dealing with real clinical practice and scenarios of prognostic uncertainty and severity, such as in critically ill patients. This is because the initial window of opportunity to act on this pathology is key to the prognosis and the natural history of this infection.
Conflicts of interestNo conflicts of interest to declare.
| SARS-CoV-2 | ||||
|---|---|---|---|---|
| ICU admission+ARDS | ||||
| Histology | Microbiology 1 | Imaging | Clinical factors | Microbiology 2 |
| Invasive growth on microscopyOrPositive cultureOrPositive PCROrCombination | BAL microscopy positiveOrBAL culture positiveOrLFA GM serum>0.5OrGM BAL>1OrX2 Positive PCR in plasma, serum or whole bloodOrPositive PCR in BAL<36 cycles*OrPositive PCR in plasma, serum or whole blood*+Positive PCR in BALOrA combination | Pulmonary infiltrateOrCavitating infiltrateOrBoth | Refractory feverOrPleural rubOrChest painOrHaemoptysisOrA combination | NBL microscopy positiveOrNBL culture positiveOrNBL GM>4.5OrX2 NBL GM>1.2OrNBL GM>1.2+NBL PCR or LFA positiveOrA combination |







![(a) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) using Log-Rank; (b) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) applying Cox regression model. Y axis: cumulative survival; X axis: days until decease. (a) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) using Log-Rank; (b) Mortality curves at 120 days (probable cases [green] vs. possible cases [pink] vs. controls [blue]) applying Cox regression model. Y axis: cumulative survival; X axis: days until decease.](https://static.elsevier.es/multimedia/0213005X/unassign/S0213005X24003021/v1_202410080946/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



