Previous study had reported the imbalance of pro- and anti-oxidants in tuberculosis (TB), which was involved in the TB progression. This study aimed to identify the key oxidative stress indicator associated with oxidative balance in patients with TB infection.
MethodsOverall, 339 participants with TB were included in this study. The oxidative balance score (OBS) was composed of scores of dietary and lifestyle factors. Trend regression analysis, generalized additive model, polynomial regression, and threshold effect analysis, were used to examine the relationship between the serum 25-hydroxyvitamin D3 25(OH)D3 and OBS. Moreover, the correlation between serum 25(OH)D3 and OBS components was explored via Pearson analysis, and mediation analysis was used to explore the function of OBS components.
ResultsLinear regression analysis showed that among 6 oxidative stress indicators, only serum 25(OH)D3 was independently related to the OBS. A positive association between them was then found (P for trend<0.05) in a non-linear relationship (all P<0.05). The 25(OH)D3 concentration of 51.9nmol/L was identified as the key turning point by threshold effect analysis, and their association was only found when it <51.9nmol/L. Further, we revealed that 25(OH)D3 mainly correlated with 2 OBS components including BMI and riboflavin. Moreover, BMI and riboflavin were found to mediate the association of serum 25(OH)D3 with OBS by mediation analysis (all P<0.05).
ConclusionsIn summary, our study revealed relationship between the serum 25(OH)D3 and OBS in patients with TB, which provided a piece of evidence that vitamin D can assist the treatment of TB.
Estudios previos han informado sobre el desequilibrio entre prooxidantes y antioxidantes en la tuberculosis (TB), implicado en la progresión de la enfermedad. El objetivo de este estudio fue identificar un indicador clave de estrés oxidativo asociado al equilibrio oxidativo en pacientes con TB.
MétodosUn total de 339 participantes con TB fueron incluidos en este estudio. La puntuación del equilibrio oxidativo (OBS) se calculó a partir de factores dietéticos y de estilo de vida. Se utilizaron análisis de regresión de tendencias, modelos aditivos generalizados, regresión polinómica y análisis de efecto umbral para examinar la relación entre la 25-hidroxivitamina D3 [25(OH)D3] sérica y la OBS. Además, se exploró la correlación entre la 25(OH)D3 sérica y los componentes de la OBS mediante análisis de Pearson, y se aplicó un análisis de mediación para investigar la función de los componentes de la OBS.
ResultadosEl análisis de regresión lineal mostró que, entre los 6 indicadores de estrés oxidativo, solo la 25(OH)D3 sérica estaba relacionada de manera independiente con la OBS. Se observó una asociación positiva entre ambos (p para la tendencia<0,05) en una relación no lineal (todas las p<0,05). Una concentración de 25(OH)D3 de 51,9nmol/L se identificó como un punto de inflexión clave mediante análisis de efecto umbral, encontrándose la asociación únicamente cuando la concentración era<51,9nmol/L. Además, se reveló que la 25(OH)D3 se correlacionaba principalmente con 2 componentes de la OBS: el IMC y la riboflavina. Asimismo, el análisis de mediación mostró que el IMC y la riboflavina mediaban la asociación entre la 25(OH)D3 sérica y la OBS (todas las p<0,05).
ConclusionesEn resumen, nuestro estudio reveló la relación entre la 25(OH)D3 sérica y la OBS en pacientes con TB, proporcionando evidencia de que la vitamina D podría ayudar en el tratamiento de la tuberculosis.
Tuberculosis (TB) is an airborne infectious disease caused by microorganisms belonging to the mycobacterium TB complex. While predominantly affecting the lungs, mycobacterium TB has the potential to cause disease in various anatomical sites.1 TB, the primary cause of mortality globally among adults due to an infectious disease, has been recognized as a critical public health crisis on a global scale for the past quarter century. In 2017, the World Health Organization (WHO) reported that approximately 10 million individuals contracted Mycobacterium tuberculosis (M.tuberculosis), with 8.7million (87%) of cases occurring in 30 high-burden countries. Despite this high number of cases, only 6.4 million individuals were officially diagnosed and reported. It is estimated that 1.3 million people succumb to TB annually.2 In 2015, the WHO released it's End TB Strategy, outlining ambitious objectives to decrease global TB incidence by 90% and mortality rates by 95% by the year 2035.3 While public health interventions have resulted in the preservation of numerous lives, advancements in the containment of TB have been limited. The emergence of drug-resistant strains of TB poses a significant threat, with projections indicating that they may become the most lethal pathogens worldwide, accounting for a significant portion of fatalities attributed to antimicrobial resistance.4,5 Research has shown that HIV infection, diabetes, history of hospitalization, poor diet quality, and illiteracy6,7 were related factors for TB. However, these factors affect the outcome of TB infection by affecting the body's oxidative stress process.8 It follows that the involvement of oxidative stress may be critical for the infection of TB and its development.
Oxidative stress is a barrier to the development of many diseases, and excessive generation of harmful reactive oxygen and nitrogen compounds can cause its imbalance.9 Abnormal oxidative stress can damage cell membranes, DNA, oxidation of proteins, cell apoptosis process, and cause other adverse effects, thereby impacting the occurrence and development of various diseases.10 Oxidative stress status has a dual effect on the M. tuberculosis. For example, M. tuberculosis responds to reactive oxygen species (ROS) in a concentration-dependent manner. Exposure to low concentrations of ROS has no effect on bacterial cell viability but induces the expression of ROS-sensitive response genes, while high concentrations exposure of ROS have been found to be lethal to M. tuberculosis cells.11 Overall, when the level of ROS produced by host cells is low, M. tuberculosis triggers the release of DNA damage response genes, which initiate DNA repair mechanisms within the bacteria to counteract ROS-induced damage.11 However, this counteraction mechanism of M. tuberculosis does not against high levels of ROS.12 Therefore, appropriate oxidative stress status is critical for the inhibition of TB development.
However, single factors cannot reflect the level of oxidative stress in the body adequately. Therefore, researchers have proposed the concept of an oxidative balance score (OBS).13 The concept of “OBS” refers to a metric used to assess the overall balance between oxidative stress and antioxidant capacity in the body. It quantifies the equilibrium between reactive oxygen species (ROS) production and the body's ability to neutralize them through antioxidant mechanisms. This score integrates various factors related to oxidative stress, such as levels of ROS, antioxidant enzymes, and oxidative damage markers, into a single numerical value, providing a comprehensive measure of the oxidative status of an individual.14,15 Most studies have shown that OBS is related to the occurrence and development of diseases. For instance, studies have shown that OBS is negatively correlated with sleep disorders and positively correlated with sleep duration.16 The results of Da-Hye Son et al. suggested that a healthy diet and lifestyle that increases the OBS may help prevent chronic kidney disease in adults.17 Higher OBS can reduce the risk of diabetes and heart disease.18,19 In TB patients, an imbalance of pro- and anti-oxidants has been observed.20 The clinical value of OBS in various diseases has been gradually reported. However, no study reported the role of oxidative stress in the OBS in TB patients.
To the best of our knowledge, the relationship between oxidative stress and OBS in TB has not been unclear. In this study, we aimed to explore their association in patients with TB infection in U.S. adults based on the National Health and Nutrition Examination Survey (NHANES) database. In addition, we also revealed the key oxidative stress indicators and OBS components that affected the oxidative balance.
Materials and methodsStudy populationNHANES is a series of nationwide population-based surveys conducted in the United States, collecting information on health and nutrition, which is released to the public every two years. Using a complex stratified sampling design, NHANES selects a representative sample of the non-institutionalized civilian population of the United States, including Mexican Americans, African Americans, and individuals of other races. The NHANES study protocol has been approved by the Institutional Review Board of the National Center for Health Statistics in the United States, and written informed consent has been obtained from all registered participants. The dataset used in this study is NHANES 2011–2012 as only this cycle has the record of TB. The components of the survey interviews used in this study include demographic characteristics, dietary data, and laboratory examination data.
The TB infection inclusion criteria were as follows: age>18 years old, had complete data of QuantiFERON-TB Gold In-Tube test (QFT-GIT). Individuals with indeterminate QFT-GIT results were excluded. A total of 9756 samples were obtained from the NHANES 2011–2012 cycle, and 7111 samples had the QFT-GIT data. After removing participants with age<18 years old, 5257 samples were retained. Then, the 51 pregnant women and 902 participants who missed the OBS data were excluded. A total of 4290 participants were further used to diagnose TB according to QFT-GIT. QFT-GIT was performed and interpreted according to the guidelines provided by the CDC for using IGRA. The three criteria were used to define positive QFT-GIT: first, Nil value must be ≤8.0IU gamma interferon (IF)/ml, second, the TB antigen value minus Nil value must be ≥0.35IU gamma interferon (IF)/ml, third, TB antigen value minus Nil value must be ≥25% of the Nil value.21 Finally, 399 patients with positive QFT-GIT results were enrolled in our analysis.
Variables collectionWe collected demographic characteristics including gender, age (years), marital status, education level, poverty status, smoking status, alcohol consumption, underlying diseases, body mass index (BMI, kg/m2), and oxidative stress indicators including Albumin (g/dL), Bilirubin (mg/dL), Uric acid (mg/dL), serum 25-hydroxyvitamin D2 (25(OH)D2, nmol/L), 25(OH)D3 (nmol/L), and gamma-glutamyl transpeptidase (GGT, U/L).
OBS was assessed according to related dietary and lifestyle factors, including dietary fiber, carotenoids (α- and β-carotene), riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total fat intake, iron intake, BMI, physical activity, and contribution to oxidative stress by antioxidants.22 In the evaluation process, higher dietary antioxidant intake and more physical activity induce an increase in OBS, indicating a stronger antioxidant capacity. Conversely, increased total fat, iron, antioxidant intake, and BMI lead to a decrease in OBS, reflecting an increase in oxidative stress levels. The final OBS is obtained by adding up the allocated scores for each component.
Statistical analysesAll statistical analyses used subsample weights following NHANES guidelines in R software version 4.2.2. The Chi-square test was used for the categorical variables and the T-test for continuous variables. The relationship between the indicators and OBS was analyzed via linear regression to obtain the indicators significantly related to OBS. We found that only serum 25(OH)D3 was significantly related to OBS. Therefore, the trend regression analysis, generalized additive model (GAM), polynomial regression, and threshold effect analysis, were used to further analyze the relationship between the serum 25(OH)D3 and OBS. In addition, the correlation between serum 25(OH)D3 and OBS components was explored via Pearson analysis, and mediation analysis was used to explore the function of OBS components that were significantly related to serum 25(OH)D3 in the relationship between serum 25(OH)D3 and OBS. All statistical tests conducted in this study were two-tailed, with the threshold for statistical significance set at P<0.05.
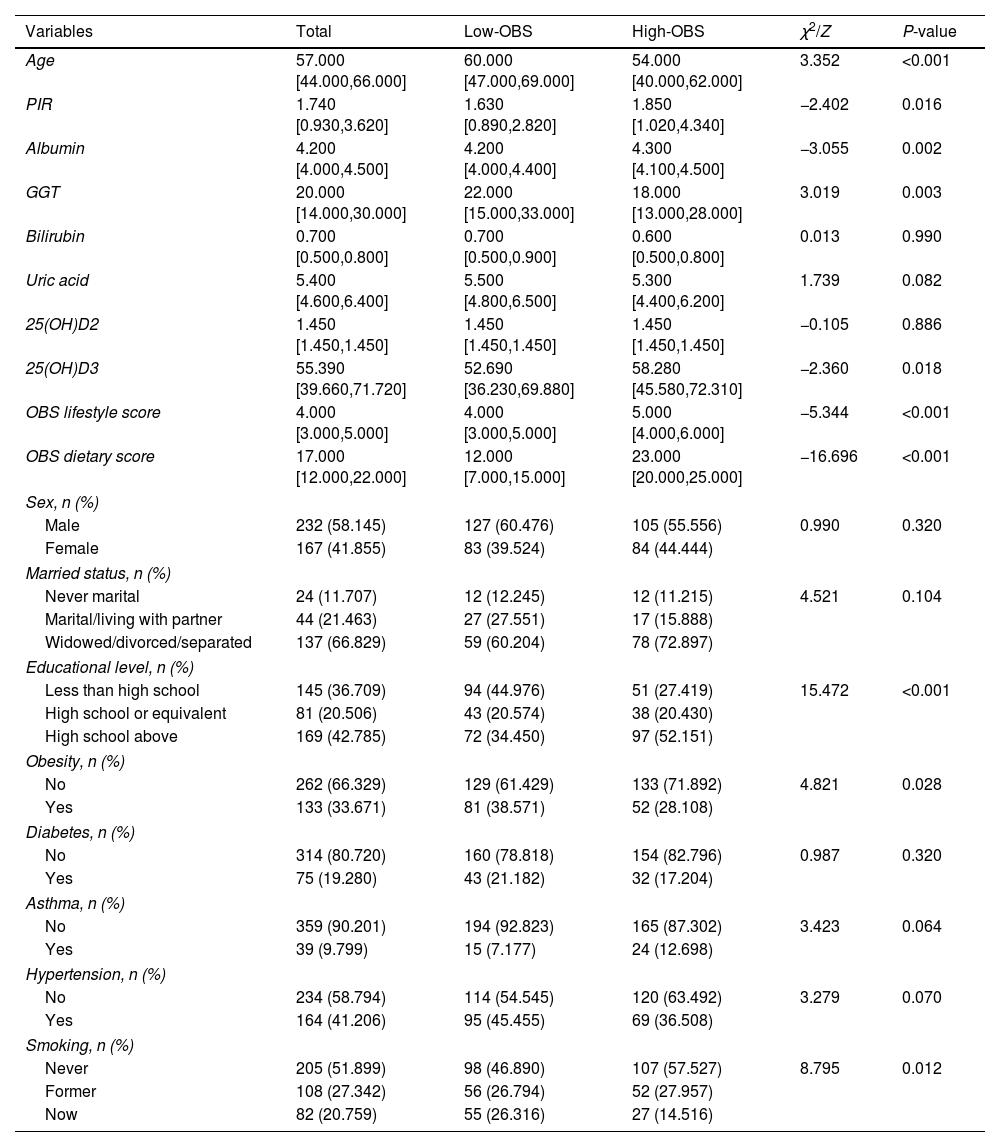
ResultsThe baseline characteristics of participantsA total of 399 QFT-GIT positive patients were enrolled in this study. Characteristics of TB patients grouped by the median value of OBS are presented in Table 1. Eight characteristics including age, PIR, albumin, GGT, serum 25(OH)D3, educational level, obesity, and smoking status all showed significant differences between the 2 groups (all P<0.05). These 8 characteristics were enrolled in our further analysis.
Analysis of baseline characteristics and oxidative stress indicators related to OBS.
| Variables | Total | Low-OBS | High-OBS | χ2/Z | P-value |
|---|---|---|---|---|---|
| Age | 57.000 [44.000,66.000] | 60.000 [47.000,69.000] | 54.000 [40.000,62.000] | 3.352 | <0.001 |
| PIR | 1.740 [0.930,3.620] | 1.630 [0.890,2.820] | 1.850 [1.020,4.340] | −2.402 | 0.016 |
| Albumin | 4.200 [4.000,4.500] | 4.200 [4.000,4.400] | 4.300 [4.100,4.500] | −3.055 | 0.002 |
| GGT | 20.000 [14.000,30.000] | 22.000 [15.000,33.000] | 18.000 [13.000,28.000] | 3.019 | 0.003 |
| Bilirubin | 0.700 [0.500,0.800] | 0.700 [0.500,0.900] | 0.600 [0.500,0.800] | 0.013 | 0.990 |
| Uric acid | 5.400 [4.600,6.400] | 5.500 [4.800,6.500] | 5.300 [4.400,6.200] | 1.739 | 0.082 |
| 25(OH)D2 | 1.450 [1.450,1.450] | 1.450 [1.450,1.450] | 1.450 [1.450,1.450] | −0.105 | 0.886 |
| 25(OH)D3 | 55.390 [39.660,71.720] | 52.690 [36.230,69.880] | 58.280 [45.580,72.310] | −2.360 | 0.018 |
| OBS lifestyle score | 4.000 [3.000,5.000] | 4.000 [3.000,5.000] | 5.000 [4.000,6.000] | −5.344 | <0.001 |
| OBS dietary score | 17.000 [12.000,22.000] | 12.000 [7.000,15.000] | 23.000 [20.000,25.000] | −16.696 | <0.001 |
| Sex, n (%) | |||||
| Male | 232 (58.145) | 127 (60.476) | 105 (55.556) | 0.990 | 0.320 |
| Female | 167 (41.855) | 83 (39.524) | 84 (44.444) | ||
| Married status, n (%) | |||||
| Never marital | 24 (11.707) | 12 (12.245) | 12 (11.215) | 4.521 | 0.104 |
| Marital/living with partner | 44 (21.463) | 27 (27.551) | 17 (15.888) | ||
| Widowed/divorced/separated | 137 (66.829) | 59 (60.204) | 78 (72.897) | ||
| Educational level, n (%) | |||||
| Less than high school | 145 (36.709) | 94 (44.976) | 51 (27.419) | 15.472 | <0.001 |
| High school or equivalent | 81 (20.506) | 43 (20.574) | 38 (20.430) | ||
| High school above | 169 (42.785) | 72 (34.450) | 97 (52.151) | ||
| Obesity, n (%) | |||||
| No | 262 (66.329) | 129 (61.429) | 133 (71.892) | 4.821 | 0.028 |
| Yes | 133 (33.671) | 81 (38.571) | 52 (28.108) | ||
| Diabetes, n (%) | |||||
| No | 314 (80.720) | 160 (78.818) | 154 (82.796) | 0.987 | 0.320 |
| Yes | 75 (19.280) | 43 (21.182) | 32 (17.204) | ||
| Asthma, n (%) | |||||
| No | 359 (90.201) | 194 (92.823) | 165 (87.302) | 3.423 | 0.064 |
| Yes | 39 (9.799) | 15 (7.177) | 24 (12.698) | ||
| Hypertension, n (%) | |||||
| No | 234 (58.794) | 114 (54.545) | 120 (63.492) | 3.279 | 0.070 |
| Yes | 164 (41.206) | 95 (45.455) | 69 (36.508) | ||
| Smoking, n (%) | |||||
| Never | 205 (51.899) | 98 (46.890) | 107 (57.527) | 8.795 | 0.012 |
| Former | 108 (27.342) | 56 (26.794) | 52 (27.957) | ||
| Now | 82 (20.759) | 55 (26.316) | 27 (14.516) | ||
Abbreviations: OBS: oxidative balance score, PIR: poverty income ratio, GGT: gamma-glutamyl transpeptidase, serum 25(OH)D2: 25-hydroxyvitamin D2, serum 25(OH)D3: serum 25-hydroxyvitamin D3, serum 25(OH)D: serum 25-hydroxyvitamin D.
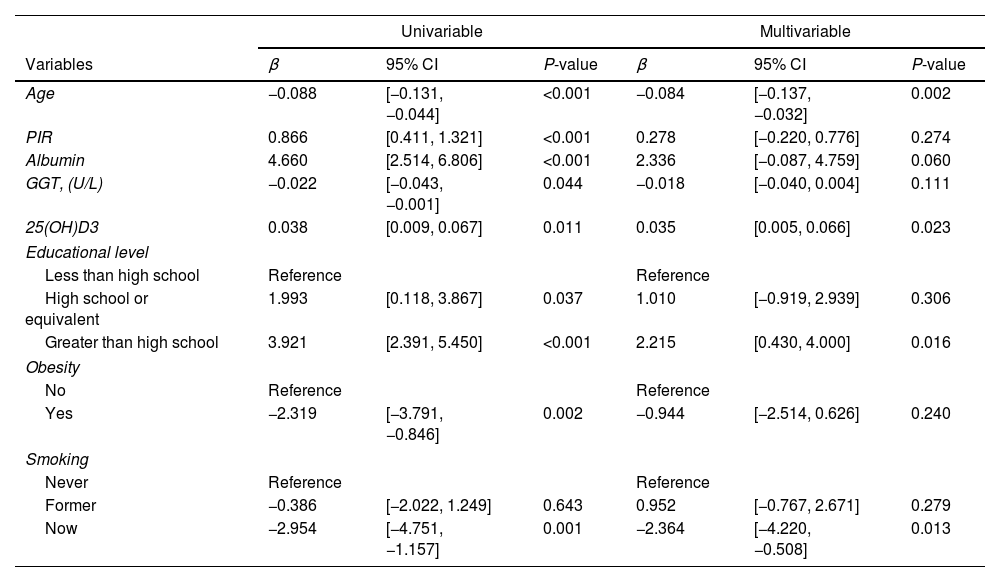
We further performed the univariable linear regression to analyze the relationship between the 8 characteristics and OBS. The results showed that all variables had significant relationships with OSB (Table 2). Therefore, those variables were further enrolled in multivariable linear regression to further screen the independent critical characteristics associated with OBS. The results showed that among 3 oxidative stress indicators, only serum 25(OH)D3 and OBS had a significant independent relationship (β=0.035, P=0.023). These results all suggested a significant association between 25(OH)D3 and OBS.
The association between the above significant indicators and OBS score by univariable and multivariable linear regression analysis in patients with TB.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | β | 95% CI | P-value | β | 95% CI | P-value |
| Age | −0.088 | [−0.131, −0.044] | <0.001 | −0.084 | [−0.137, −0.032] | 0.002 |
| PIR | 0.866 | [0.411, 1.321] | <0.001 | 0.278 | [−0.220, 0.776] | 0.274 |
| Albumin | 4.660 | [2.514, 6.806] | <0.001 | 2.336 | [−0.087, 4.759] | 0.060 |
| GGT, (U/L) | −0.022 | [−0.043, −0.001] | 0.044 | −0.018 | [−0.040, 0.004] | 0.111 |
| 25(OH)D3 | 0.038 | [0.009, 0.067] | 0.011 | 0.035 | [0.005, 0.066] | 0.023 |
| Educational level | ||||||
| Less than high school | Reference | Reference | ||||
| High school or equivalent | 1.993 | [0.118, 3.867] | 0.037 | 1.010 | [−0.919, 2.939] | 0.306 |
| Greater than high school | 3.921 | [2.391, 5.450] | <0.001 | 2.215 | [0.430, 4.000] | 0.016 |
| Obesity | ||||||
| No | Reference | Reference | ||||
| Yes | −2.319 | [−3.791, −0.846] | 0.002 | −0.944 | [−2.514, 0.626] | 0.240 |
| Smoking | ||||||
| Never | Reference | Reference | ||||
| Former | −0.386 | [−2.022, 1.249] | 0.643 | 0.952 | [−0.767, 2.671] | 0.279 |
| Now | −2.954 | [−4.751, −1.157] | 0.001 | −2.364 | [−4.220, −0.508] | 0.013 |
Abbreviations: OBS: oxidative balance score, GGT: gamma-glutamyl transpeptidase, CI: confidence interval, serum 25(OH)D3: serum 25-hydroxyvitamin D.
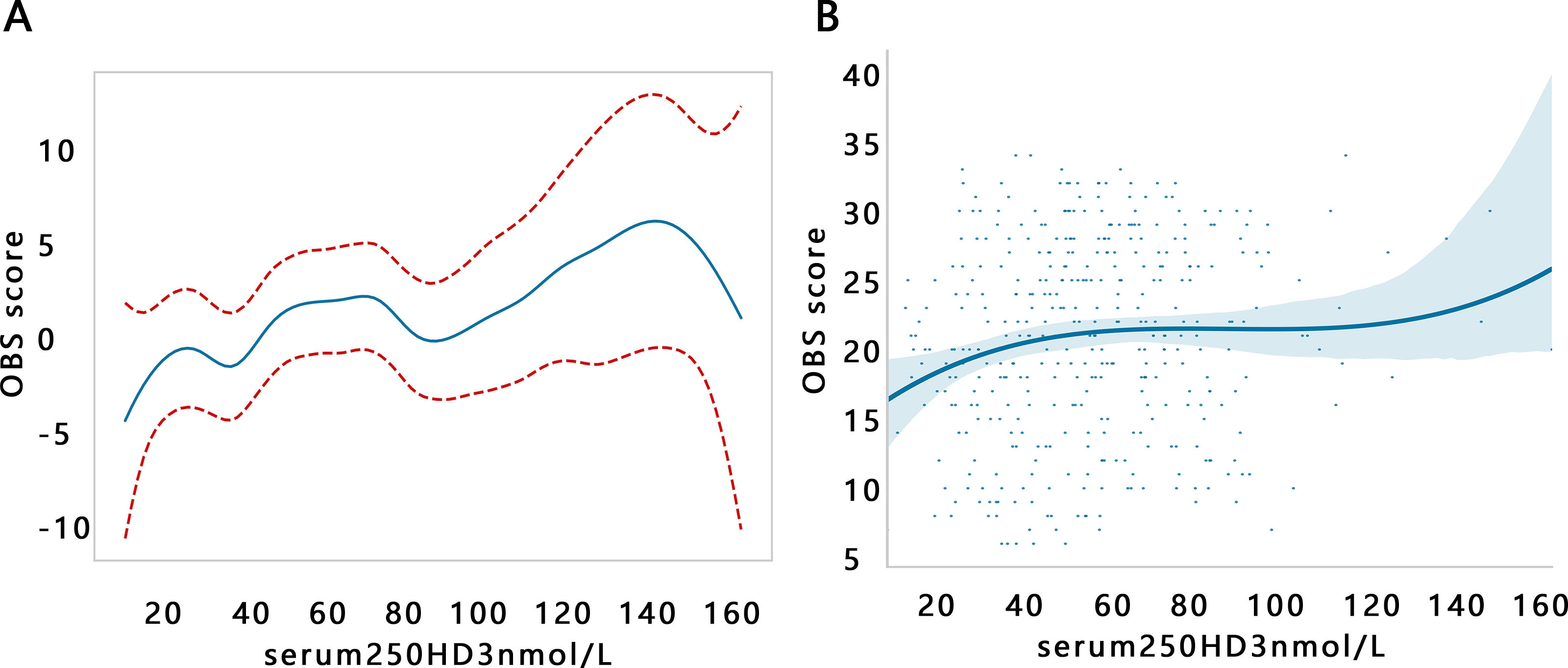
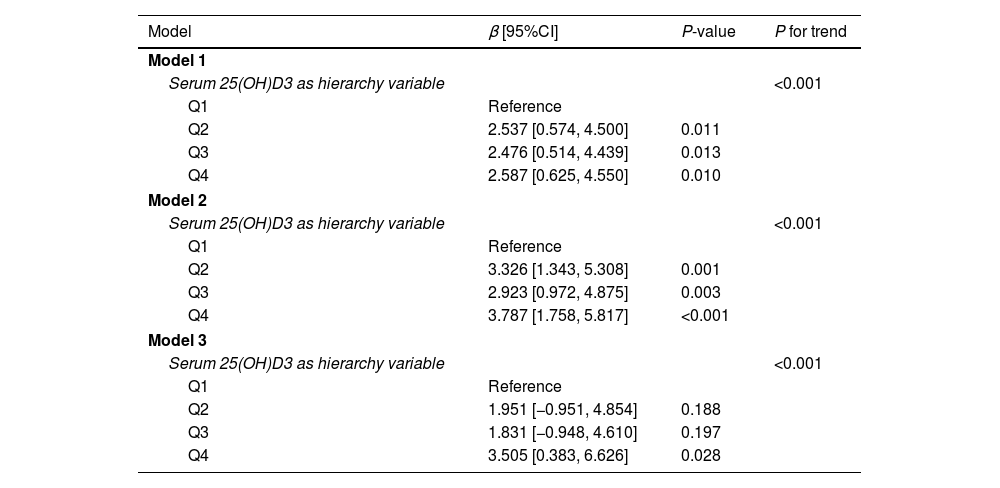
Further, we explored the association changes between serum 25(OH)D3 and OBS by trend regression analysis after setting the serum 25(OH)D3 as a hierarchy variable (Table 3). The results illustrated that with the increase of serum 25(OH)D3 level, the OBS was increased. The same results were observed among 3 models (all P for trend<0.05). However, we found that the changes of β value were not stable from Q1 to Q4 groups, and its changes appeared a fluctuant phenomenon in Q3 groups among 3 models. Therefore, we further visualized the relationship between serum 25(OH)D3 and OBS by GAM analysis. The results showed that there was a non-linear correlation of serum 25(OH)D3 with OBS (Fig. 1A, P<0.01). Consistent results are obtained by polynomial regression analysis (Fig. 1B). Their non-linear correlation may support the fluctuation of β value.
Trend regression analysis was between serum 25(OH)D3 and OBS score.
| Model | β [95%CI] | P-value | P for trend |
|---|---|---|---|
| Model 1 | |||
| Serum 25(OH)D3 as hierarchy variable | <0.001 | ||
| Q1 | Reference | ||
| Q2 | 2.537 [0.574, 4.500] | 0.011 | |
| Q3 | 2.476 [0.514, 4.439] | 0.013 | |
| Q4 | 2.587 [0.625, 4.550] | 0.010 | |
| Model 2 | |||
| Serum 25(OH)D3 as hierarchy variable | <0.001 | ||
| Q1 | Reference | ||
| Q2 | 3.326 [1.343, 5.308] | 0.001 | |
| Q3 | 2.923 [0.972, 4.875] | 0.003 | |
| Q4 | 3.787 [1.758, 5.817] | <0.001 | |
| Model 3 | |||
| Serum 25(OH)D3 as hierarchy variable | <0.001 | ||
| Q1 | Reference | ||
| Q2 | 1.951 [−0.951, 4.854] | 0.188 | |
| Q3 | 1.831 [−0.948, 4.610] | 0.197 | |
| Q4 | 3.505 [0.383, 6.626] | 0.028 | |
Model 1: no adjustment.
Model 2: adjusted for age and PIR.
Model 3: adjusted for age, PIR, educational level, obesity, and smoking.
Abbreviations: OBS: oxidative balance score, CI: confidence interval, serum 25(OH)D3: serum 25-hydroxyvitamin D3.
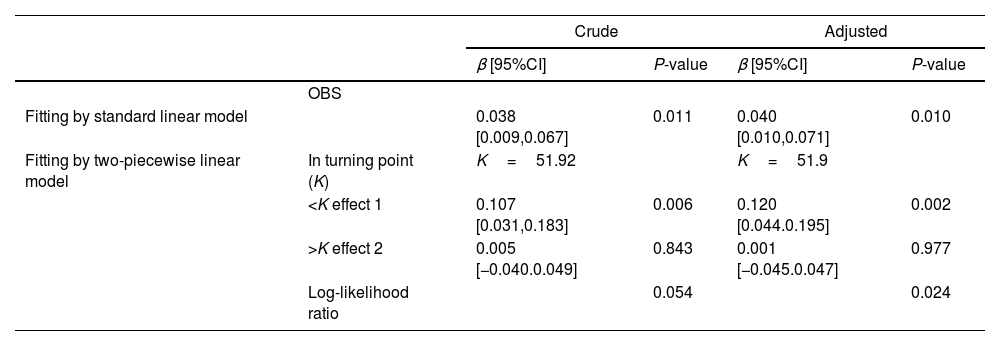
Then, the potential inflection point influencing their association was explored through the threshold effect analysis. The results indicated that serum 25(OH)D3 was significantly related to OBS when it <51.92 (nmol/L) in the crude model, while serum 25(OH)D3 was not related to OBS when it >51.92 (nmol/L) (Table 4). A similar inflection point (51.9) was also found in the adjusted model.
Threshold effect analysis of OBS on serum 25(OH)D3.
| Crude | Adjusted | ||||
|---|---|---|---|---|---|
| β [95%CI] | P-value | β [95%CI] | P-value | ||
| OBS | |||||
| Fitting by standard linear model | 0.038 [0.009,0.067] | 0.011 | 0.040 [0.010,0.071] | 0.010 | |
| Fitting by two-piecewise linear model | In turning point (K) | K=51.92 | K=51.9 | ||
| <K effect 1 | 0.107 [0.031,0.183] | 0.006 | 0.120 [0.044.0.195] | 0.002 | |
| >K effect 2 | 0.005 [−0.040.0.049] | 0.843 | 0.001 [−0.045.0.047] | 0.977 | |
| Log-likelihood ratio | 0.054 | 0.024 | |||
Crude: without adjustment.
Adjusted: adjusting for age, PIR, educational level, smoking, diabetes, hypertension.
Abbreviations: OBS: oxidative balance score, CI: confidence interval, serum 25(OH)D3: serum 25-hydroxyvitamin D3.
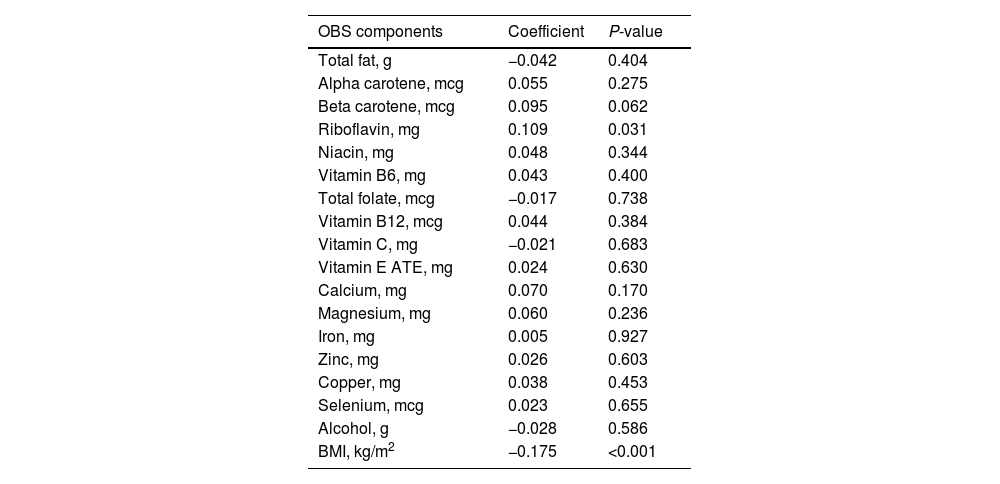
We found that the serum 25(OH)D3 was significantly related to OBS via the above results. Therefore, we further explored the relationship between the serum 25(OH)D3 and 20 components of OBS. The Pearson analysis indicated that the association of serum 25(OH)D3 with BMI and riboflavin was significant (P<0.05, Table 5).
Correlation between 25(OH)D3 and OBS components.
| OBS components | Coefficient | P-value |
|---|---|---|
| Total fat, g | −0.042 | 0.404 |
| Alpha carotene, mcg | 0.055 | 0.275 |
| Beta carotene, mcg | 0.095 | 0.062 |
| Riboflavin, mg | 0.109 | 0.031 |
| Niacin, mg | 0.048 | 0.344 |
| Vitamin B6, mg | 0.043 | 0.400 |
| Total folate, mcg | −0.017 | 0.738 |
| Vitamin B12, mcg | 0.044 | 0.384 |
| Vitamin C, mg | −0.021 | 0.683 |
| Vitamin E ATE, mg | 0.024 | 0.630 |
| Calcium, mg | 0.070 | 0.170 |
| Magnesium, mg | 0.060 | 0.236 |
| Iron, mg | 0.005 | 0.927 |
| Zinc, mg | 0.026 | 0.603 |
| Copper, mg | 0.038 | 0.453 |
| Selenium, mcg | 0.023 | 0.655 |
| Alcohol, g | −0.028 | 0.586 |
| BMI, kg/m2 | −0.175 | <0.001 |
Abbreviations: OBS: oxidative balance score, GGT: gamma-glutamyl transpeptidase, BMI: body mass index, serum 25(OH)D3: serum 25-hydroxyvitamin D3.
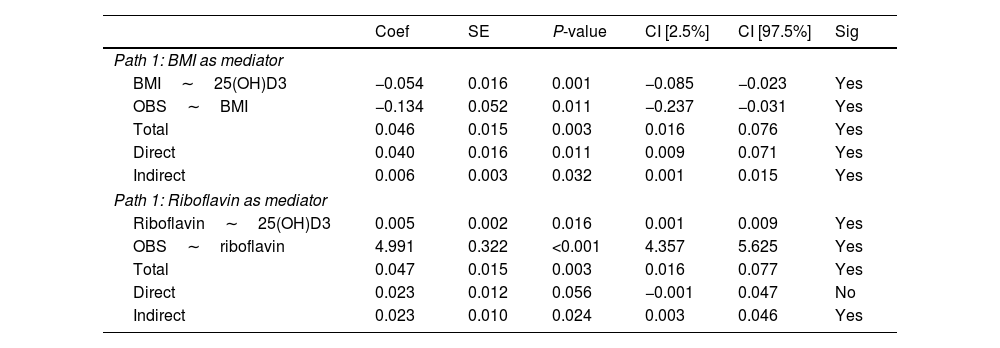
Next, we explored the effect of the BMI and riboflavin on the relationship between the serum 25(OH)D3 and OBS via mediation analysis. The results indicated that BMI and riboflavin had mediating effects on the association of serum 25(OH)D3 with OBS (all P<0.05 for the indirect effect, Table 6).
Mediation analysis by setting BMI and riboflavin as a mediator.
| Coef | SE | P-value | CI [2.5%] | CI [97.5%] | Sig | |
|---|---|---|---|---|---|---|
| Path 1: BMI as mediator | ||||||
| BMI∼25(OH)D3 | −0.054 | 0.016 | 0.001 | −0.085 | −0.023 | Yes |
| OBS∼BMI | −0.134 | 0.052 | 0.011 | −0.237 | −0.031 | Yes |
| Total | 0.046 | 0.015 | 0.003 | 0.016 | 0.076 | Yes |
| Direct | 0.040 | 0.016 | 0.011 | 0.009 | 0.071 | Yes |
| Indirect | 0.006 | 0.003 | 0.032 | 0.001 | 0.015 | Yes |
| Path 1: Riboflavin as mediator | ||||||
| Riboflavin∼25(OH)D3 | 0.005 | 0.002 | 0.016 | 0.001 | 0.009 | Yes |
| OBS∼riboflavin | 4.991 | 0.322 | <0.001 | 4.357 | 5.625 | Yes |
| Total | 0.047 | 0.015 | 0.003 | 0.016 | 0.077 | Yes |
| Direct | 0.023 | 0.012 | 0.056 | −0.001 | 0.047 | No |
| Indirect | 0.023 | 0.010 | 0.024 | 0.003 | 0.046 | Yes |
Abbreviations: OBS: oxidative balance score, BMI: body mass index, CI: confidence interval, serum 25(OH)D3: serum 25-hydroxyvitamin D3.
A previous study has reported the imbalance of pro- and anti-oxidants in TB.20 In this study, we further explored the important oxidative stress indicators that correlated with the oxidative balance in TB. Among 6 oxidative stress indexes, only serum 25(OH)D3 showed a significant association with the OBS in patients with TB infection. To delve deeper into this relationship, the threshold effect analysis was performed and its results indicated that serum 25(OH)D3 was positively related to OBS when serum 25(OH)D3<51.9nmol/L in the patients with TB. To our knowledge, this is the first study to explore the association between serum 25(OH)D3 and OBS in patients with TB. Our study initially suggested the importance of 25(OH)D3 on positive regulation of oxidative balance in TB.
Currently, few studies reported their potential association. However, studies have indicated that there was a slight correlation between the levels of serum 25(OH)D3 and the outcomes of TB, and higher concentrations of serum 25(OH)D3 was associated with higher cure rates.23 According to the positive association between serum 25(OH)D3 and OBS in this study, we speculated that the increased cure rates associated with higher serum 25(OH)D3 level may be related to the increase of OBS. OBS is a scoring index based on the calculation of dietary and lifestyle-promoting and antioxidant components,22 and always used to assess systemic oxidative stress status. Higher OBS scores implied exposure to more antioxidants.24 Therefore, higher serum 25(OH)D3 level may refer to a stronger antioxidant environment in TB patients. A study reported that the epigallocatechin gallate (a phenolic antioxidant) in green and black tea can decrease the risk of contracting TB.25 Ginger was an effective antioxidant supplement along with anti-TB therapy, as it possesses strong free radical scavenging property.26 These knowledges may partially explain the favorable effect of serum 25(OH)D3 on the outcome of TB patients by inducing antioxidant environment and scavenging free radical.
Besides the regulation on the oxidative stress environment in TB, serum 25(OH)D3 can also mediate immune responses against M. tuberculosis, inducing the production of methyl ethyl ketone and β-defensin 2 (antimicrobial peptides), recruiting monocytes, neutrophils, and T cells to the site of infection.27 Granulysin in T cells is a cationic, amphiphilic, low-molecular-weight antimicrobial peptide that functions to damage cell walls, exhibiting antimicrobial activity against various microbial pathogens, promoting the permeabilization of mycobacteria, and reducing growth in a perforin-dependent manner,28,29 thereby inhibiting the progression of TB and consequently improving the OBS of patients with TB.
Further, we identified 2 key OBS components associated with the serum 25(OH)D3, namely BMI and riboflavin. The serum 25(OH)D3 showed negative correlation with pro-oxidant factor BMI but displayed positive correlation with antioxidant factor riboflavin. Existing studies have demonstrated an association between BMI and vitamin D levels.30,31 For example, in patients with Ulcerative Colitis, an increase in BMI was associated with a 12% increased likelihood of developing serum 25(OH)D3 deficiency, as indicated by Pallav and ESPEN guidelines.32 According to the research by Vimaleswarns and Pereira-Santos, there was a direct relationship between obesity and 25(OH)D3 deficiency.33,34 The specific mechanism needs further study in the future. Our results further supported the establishment of antioxidant environment by serum 25(OH)D3. In addition, we also found that BMI and riboflavin mediated the association of serum 25(OH)D3 with OBS, which highlighted the importance of BMI and riboflavin on the oxidative balance in TB.
This study revealed the relationship between the serum 25(OH)D3 and the OBS in patients with TB for the first time but also had some limitations. First, due to all the components used in the OBS being equally weighted, they may not accurately represent the actual biological contributions. Second, the result was not an exact causal relationship because of cross-sectional analysis. Third, TB patients diagnosed by other methods may be omitted. Fourth, the population was collected from the NHANES database, which did not represent the global population. Fifth, this study only included important indicators that we collected from the literature, and there may be omissions of indicators that affect serum 25(OH)D3 levels, which may lead to biased results.
ConclusionsIn summary, serum 25(OH)D3 was significantly related to OBS in the patients with TB, and their association was especially significant when serum 25(OH)D3 level<51.9 (nmol/L). Moreover, BMI and riboflavin were the key OBS components correlated with serum 25(OH)D3, and they significantly mediated the association of serum 25(OH)D3 with OBS in patients with TB.
Authors’ contributionsYan Yong contributed to the conception and design. Yan Yong and Li-hong Zhou, Sheng-ya Yang contributed to the collection and assembly of data. Yan Yong, Xiao-qin Ran and Qing-shan Cai analyzed and interpreted the data. All authors wrote and approved the final manuscript.
FundingNot applicable.
Conflict of interestThe authors declare no conflict of interest.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.