A 67-year-old woman who received surgery, radiotherapy, and chemotherapy six months ago for the treatment of lung adenoid cystic carcinoma (ACCM) was admitted to our hospital with a ten-day history of cough and shortness of breath. She was admitted 17 days after her last round of chemotherapy, during which she developed fever and leucopenia, which improved after treatment. Two days before admission, she experienced shortness of breath and went to a local hospital. Despite treatment, the dyspnea persisted.
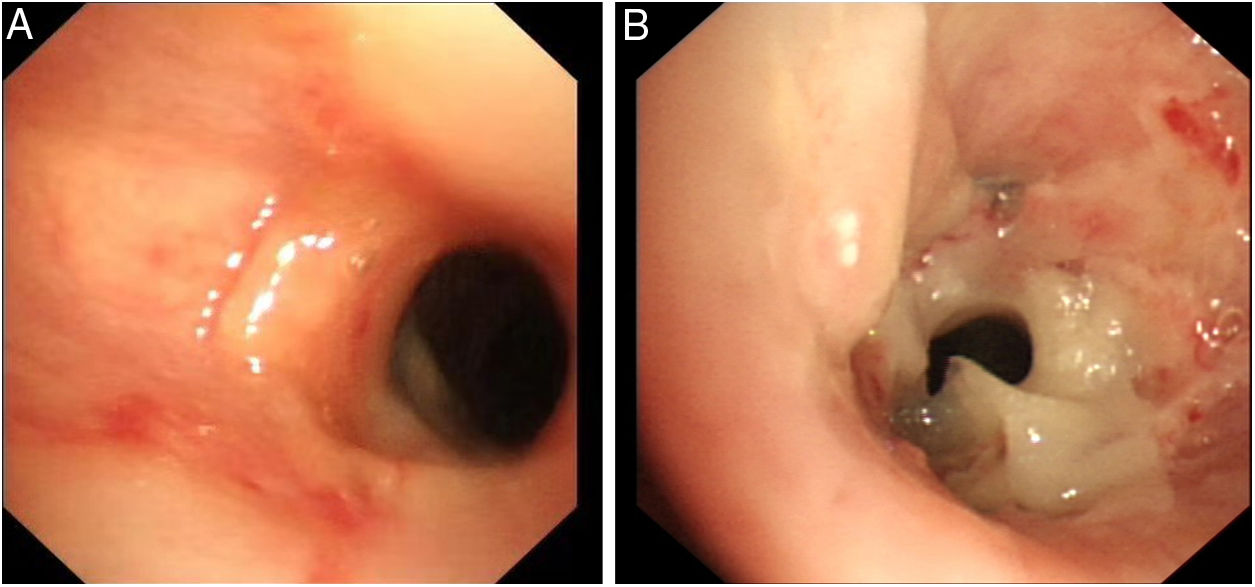
After admission to our hospital, a pulmonary examination revealed mild cyanosis, signs of respiratory depression and diminished breath sounds in the left upper lobe. Laboratory tests showed a white blood cell (WBC) count of 17.88×109L-1 (86.6% neutrophils). Sputum cultures were negative before the implantation of bronchial stent. Chest computed tomography (CT) revealed a low-density shadow in left bronchial cavity, left upper atelectasis, and right-sided pneumonia. The patient was initially treated with moxifloxacin and cefotaxime due to elevated WBC and right-sided pneumonia. Meanwhile, she received a fluorescence bronchoscopy for the left upper lobe atelectasis detected through CT. Bronchoscopy revealed prominent scar tissue stenoses in the middle trachea and left main bronchus. The patient was implanted with two metallic bronchial stents at these two locations and her condition improved subsequently. However, she experienced shortness of breath again three days later. A second bronchoscopy revealed a concentration of necrotic pseudo-membranes at the top of the stent (Fig. 1A). Removing the obstructive biofilm relieved the symptoms. One week later, however, the symptoms recurred and could not be alleviated. A third bronchoscopy revealed a large number of pseudo-membranes clustered at the top of the stent, narrowing the lumen (Fig. 1B). Dyspnea was relieved once the necrotic pseudo-membranes were removed. The necrotic specimen was sent to the laboratory and Corynebacterium striatum was detected through blood agar plate, Gram staining, and VITEK 2 Compact automatic microbial analysis system. Since C. striatum is considered to be a pathogenic bacterium, she was treated with the empirical therapy of vancomycin (1g every 12h) for 14 days. Dyspnea resolved, and she had no recurrence of symptoms. She was re-admitted to the hospital for evaluation at one month and three months post-treatment. Bronchoscopies at both follow-ups showed no stent obstruction or new formation of necrotic pseudo-membranes. Furthermore, both bronchoalveolar lavage cultures were negative.
C. striatum, which belongs to Corynebacterium spp, is a part of the normal flora of the human skin and respiratory tract. Although commonly considered a contaminant, it has been increasingly reported as an etiologic agent of various infections, and commonly infects the respiratory tract. C. striatum was first reported in 1980 as a pathogenic bacteria causing lung infection in a patient with chronic lymphocytic leukemia.1 Renom et al. reported the first nosocomial outbreak of C. striatum in patients with chronic obstructive pulmonary disease in 2007.2 Additionally, Roig-Rico et al. described C. striatum pneumonia in an HIV patient in 2011.3 The aforementioned patients were all with severe underlying diseases. In our case, the patient was immunocompromised due to radiotherapy and chemotherapy after surgery for ACCM. Los-Arcos et al. reported that the incidence of Corynebacterium detection was significantly higher in patients with a bronchial stent than in those without (29% versus 4%) and C. striatum was the main isolated species (48%).4 However, the average period for the Corynebacterium respiratory isolation after stent implantation was 1036 days.4 A recent study revealed C. striatum played a central role in endobronchial stent biofilm structure and was not generally considered a pathogenic bacteria.5 However, our patient developed dyspnea within three days of stent implantation and the symptoms recurred in the next few days, indicating that C. striatum was a pathogenic bacterium rather than a contaminant.
C. striatum is generally resistant to penicillin but susceptible to other β-lactams and vancomycin. However, recent studies show C. striatum is resistant to a variety of antibiotics including daptomycin, macrolides, and cephalosporins.6,7 In fact, Los-Arcos et al. reported that 16.3% isolates were sensitive only to vancomycin.4 Hahn et al. reported that the minimum inhibitory concentration (MIC) distribution for vancomycin for C. striatum isolates was 0.6mg/L, which was universally active in vitro and could be used as empirical therapy.8 Topic et al. suggested a need for a prolonged antibiotic treatment (6 weeks) in patients with fever and bacteremia.9 Fortunately, our patient had only respiratory symptoms and did not present with fever. She was treated with vancomycin for 14 days. The follow-up results of the patient were satisfactory.
In conclusion, our case indicates that C. striatum can be a pathogenic bacterium in patients with stent implantation and should be taken seriously in clinical practice.