We report the case of a 43-year-old patient with a history of osteoporosis, bronchial asthma, inflammatory arthritis of unknown origin, peripheral vascular insufficiency and immunosuppression due to prolonged use of corticosteroids.
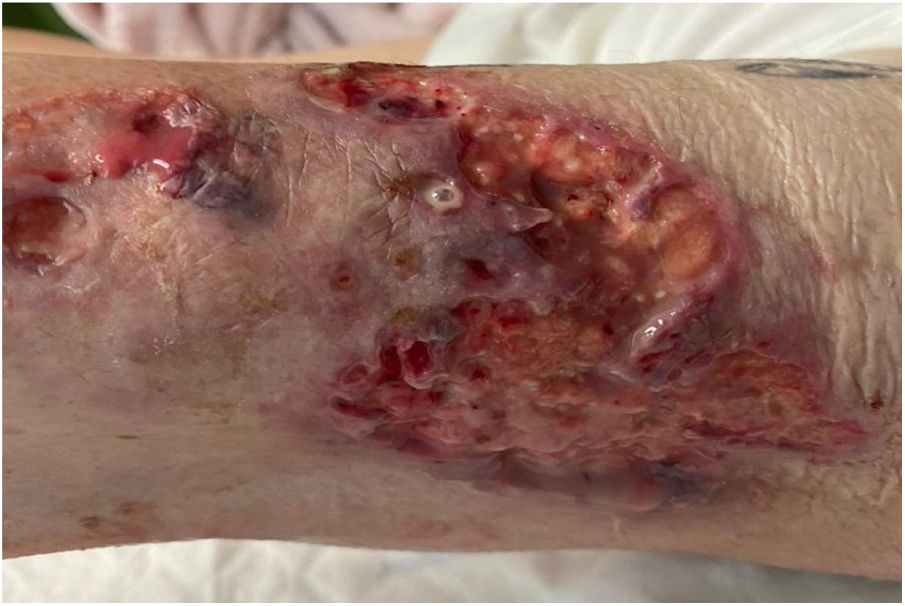
She first visited the accident and emergency department with several very painful ulcerous lesions of various sizes in a sporotrichoid pattern on her left leg, with local heat and erythema. She was diagnosed with cellulitis and prescribed amoxicillin/clavulanic acid. The patient returned to the accident and emergency department one week later due to increased pain, heat and erythema in the area of the lesions that prevented her from walking. The lesions had increased in size and were draining a purulent discharge (Fig. 1). As the patient’s lesions had worsened and her acute-phase reactant levels (C-reactive protein [CRP] and leukocytes) had increased, she was admitted and initially treated with clindamycin and ciprofloxacin. Two days later, owing to nausea, she was switched to amoxicillin/clavulanic acid and ciprofloxacin. At that time, samples were collected for bacteriological analysis. These samples were seeded in standard culture media together with thioglycolate enrichment broth. The patient’s skin samples were negative for both aerobic and anaerobic bacteria after 72 h of incubation.
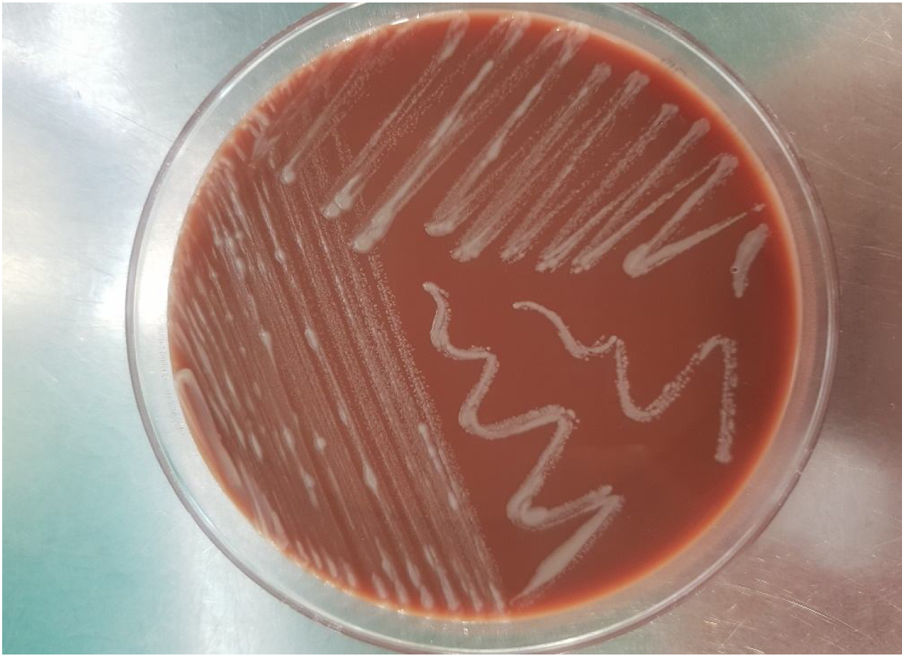
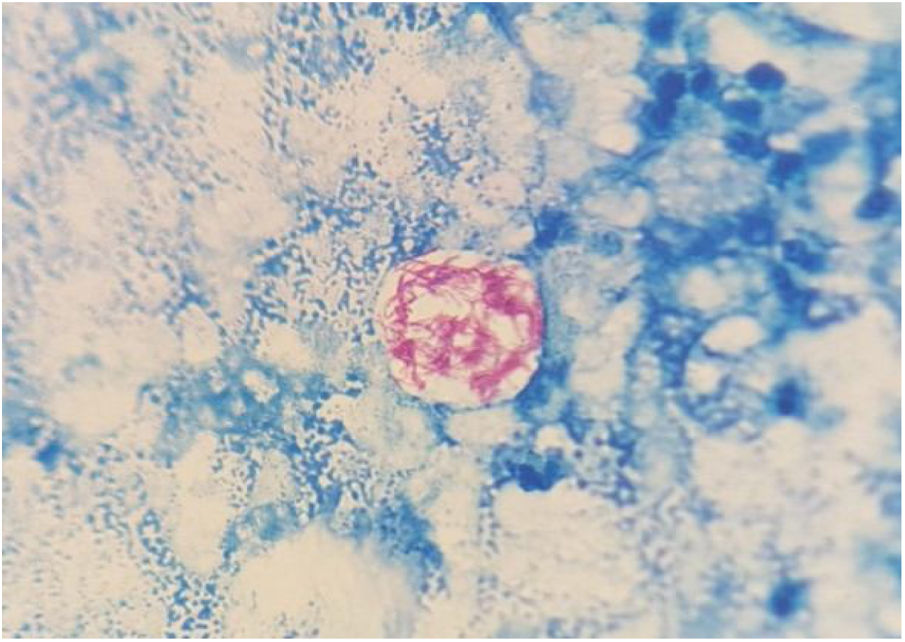
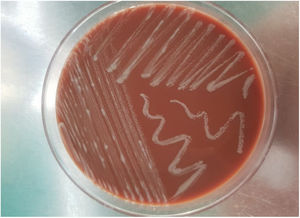
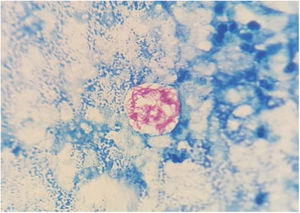
On the fifth day of incubation, the enriched medium became cloudy, so a subculture on a solid medium was performed. After 48 h, colonies with a bright and creamy appearance grew in blood agar and chocolate agar (Fig. 2). They were identified as Gram-positive Mycobacterium abscessus subsp. massiliense by Gram staining, with a score of 2.05 by mass spectrometry (MALDI-TOF MS [Bruker®]). This identification was confirmed by a 23S rRNA-based polymerase chain reaction (PCR) assay (GenoType® NTM-DR, Hain Lifescience). The sample was then recovered and subjected to Ziehl–Neelsen staining, in which acid/alcohol-fast bacilli were observed (Fig. 3). The susceptibility study was conducted by the epsilometer test (E-test) (Biomerieux®) in Mueller–Hinton agar with a 1.0 McFarland suspension, and by broth microdilution (Microscan WalkAway®, Beckman Coulter). The results showed susceptibility (according to Clinical and Laboratory Standards Institute [CLSI] criteria) to clarithromycin (minimum inhibitory concentration [MIC] = 0.016 mg/l), amikacin (MIC = 4 mg/l) and linezolid (MIC = 3 mg/l). The strain was sent to the Spanish National Centre for Microbiology (Majadahonda), where the identification and antimicrobial susceptibility were confirmed.
Correct identification of this microorganism is important as the subspecies M. abscessus subsp. abscessus possesses the erm gene, which can confer resistance to macrolides. In order to confirm that the isolate did not exhibit induction of resistance due to the presence of methylases, the antimicrobial susceptibility tests were reincubated for 14 days.
Clinical courseFollowing isolation and identification of the mycobacterium, the patient was scheduled for an appointment and prescribed clarithromycin and linezolid. After six weeks of targeted therapy, the patient’s lesions improved significantly: they were smaller, with less heat and erythema and no suppuration, and the patient tolerated the treatment better.
Closing remarksCellulitis is an acute infection that spreads gradually and deeply through the skin, affecting the subcutaneous tissue. Streptococcus pyogenes and Staphylococcus aureus are the most common bacterial causes, but the aetiology can be highly varied.1 Rapidly growing mycobacteria are pleomorphic, aerobic, immobile, intracellular bacilli resistant to environmental conditions. They are found in the environment all over the world and can cause disease in cases of chronic lesions, injections and surgical or traumatic wounds exposed to water or contaminated products.2 Most skin lesions caused by Mycobacterium chelonae or M. abscessus complex manifest with widespread painful and reddish ulcers that can affect the limbs and drain spontaneously. These infections are most common in immunosuppressed patients and do not seem to have an obvious entry point.3 The M. abscessus group consists of three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense and M. abscessus subsp. bolletii.4,5 Normally, they are resistant to fluoroquinolones and sensitive to macrolides, imipenem, amikacin and tigecycline, which can be used as an alternative therapy.6
We would like to stress the importance of clinically suspecting infection with rapidly growing mycobacteria in skin and soft-tissue lesions that do not improve with standard treatment and of extending incubation times from seven to 10 days.7,8 Correct and rapid identification by mass spectrometry is also very important.9,10
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Dr María Soledad Jiménez-Pajares of the Spanish National Centre for Microbiology (Carlos III Health Institute [ISCIII], Majadahonda, Madrid) for her help in the molecular characterisation of the mycobacterium.
Please cite this article as: Carmona-Tello MN, Hernández-Cabrera M, Lavilla-Salgado MC, Bolaños-Rivero M. Celulitis abcesificada con patrón esporotricoide que no cede al tratamiento antibiótico. Enferm Infecc Microbiol Clin. 2021;39:411–412.