Antimicrobial defined daily dose (DDD), has limitations for antimicrobial consumption measurement in paediatrics. An alternative DDD design applicable for children is proposed.
MethodsChildren (<16 years-old) from 10 Spanish hospitals during a 12-months period were included. Weight for age (50th percentile) was calculated for the median age of the cohort using standardized World Health Organization tables. DDD (g) for each antimicrobial was calculated by multiplying the obtained weight times the recommended dose (mg/kg) of the antimicrobial for the most common infectious indication.
ResultsA total of 40,575 children were included. Median age was 4.17 (IQR: 1.36–8.98) and 4.81 (IQR: 1.42–9.60) years for boys and girls, respectively. Mean weight for this age was 17.08kg. Standardized DDD for representative antimicrobials were calculated.
ConclusionsA useful method for antimicrobial DDD measurement in paediatrics has been proposed and should be validated in future studies for its use in paediatric antimicrobial stewardship programmes.
La dosis diaria definida (DDD), tiene limitaciones para la medición del consumo antimicrobiano en pediatría. Se propone un diseño aplicable en niños.
MétodosSe incluyeron niños (<16 años) de 10 hospitales españoles durante un periodo de 12 meses. A partir de la mediana de edad de la cohorte, utilizando tablas estandarizadas de la OMS, se obtuvo el peso correspondiente al percentil P50 de esa edad. Se calculó la DDD (gr) multiplicando el peso obtenido por la dosis recomendada (mg/kg) de cada antimicrobiano para su indicación más común.
ResultadosUn total de 40575 niños fueron incluidos. La mediana de edad fue 4,17 (RIQ: 1,36–8,98) y 4,81 (RIQ: 1,42–9,60) años para niños y niñas, respectivamente. Peso medio para la edad: 17,08kg. DDD estandarizadas fueron calculadas para antimicrobianos representativos.
ConclusionesSe ha propuesto un método útil para monitorizar consumo antimicrobiano en pediatría utilizando DDD adaptadas, que deberá validarse en futuros estudios.
Antimicrobials are among the most commonly prescribed drugs in paediatrics. However, up to 50% of in-hospital antimicrobial prescriptions are inappropriate.1 The relationship between inadequate use of antimicrobials and the emergence of bacterial resistance has been clearly established, resulting in increased mortality and costs.2 Antimicrobial stewardship programmes (ASP) were successfully implemented to optimize antimicrobial use in hospitalized patients.3 Though, development of ASP in paediatrics has been limited, due in part to the lack of a standardized method for comparing antimicrobial use.4
Defined Daily Dose (DDD) is one of the established metrics used by ASP, allowing the assessment of antimicrobial consumption. The World Health Organization (WHO) expresses DDD as the average standard daily dose of a drug used in a 70kg adult for the most common indication.5,6 However, the validity of DDD WHO definition is questionable in hospitalized children, in which dosing is based on body weight.1,5 This study aims to establish a methodology for antimicrobial DDD measurement in the paediatric population.
MethodsData collectionAn observational retrospective study was performed. Children from 10 Spanish hospitals (9 tertiary and 1 secondary) aged 1 month to 16 years old, with at least one episode of hospital admission into a paediatric ward, whether receiving antibiotics or not, during a 12-months period (January to December 2013) were included. Each patient hospital admission was considered an “episode”. For the study purposes, different hospital admissions through the study period and/or change of in-hospital ward during the same hospital admission period were considered as different episodes. Studied variables were age and sex, obtained through the hospitals’ admission records.
Data analysisMean age, median and range were calculated for each sex. Weight (kg) for DDD calculation was selected for the obtained median age by sex using the 50th percentile according to the WHO weight for age graphs in paediatrics.7 Overall cohort weight was the mean between the female and male selected weight values. Finally, paediatric DDD (g) for each antimicrobial was calculated through the multiplication of the overall cohort weight (kg) and the recommended dose for the most common indication of each antimicrobial (mg/kg) previously agreed.
The Delphi method was used to find a joint agreement for antimicrobial dose discrepancies. This method is a structured process that uses a series of questionnaires or “rounds” to gather information, which are held until group consensus is reached.8 Agreement process was as follows: First, one pharmacist and one paediatric infectious diseases specialist from each participating centre established the recommended dose for the most common indication for each antimicrobial. In the second round, these doses were anonymously sent to each of the 20 experts who were asked to review again their proposed dose. In case of disagreement after this round, the antimicrobial dose was established using the database from the Medicines Committee of the Spanish Association of Paediatrics (Pediamecum).9 Agreement percentage was calculated using as numerator the number of hospitals that selected the agreed dose and as denominator the number of hospitals that proposed a dose. Data analysis was performed using SPSS statistical software, version 19 (IBM SPSS, Armonk, New York).
EthicsThe study was approved by the Spanish Agency for Medicines and Sanitary Products. It was classified as “Post-authorization study with other designs different from prospective design” on May 11th, 2015 (ID number: GAT-TEI-2015-01). Subsequently, it was approved by the Hospital Universitario Virgen del Rocio and Hospital Universitario Virgen Macarena Ethics Committee on October 24th, 2016, (ID number: 0620-N-15).
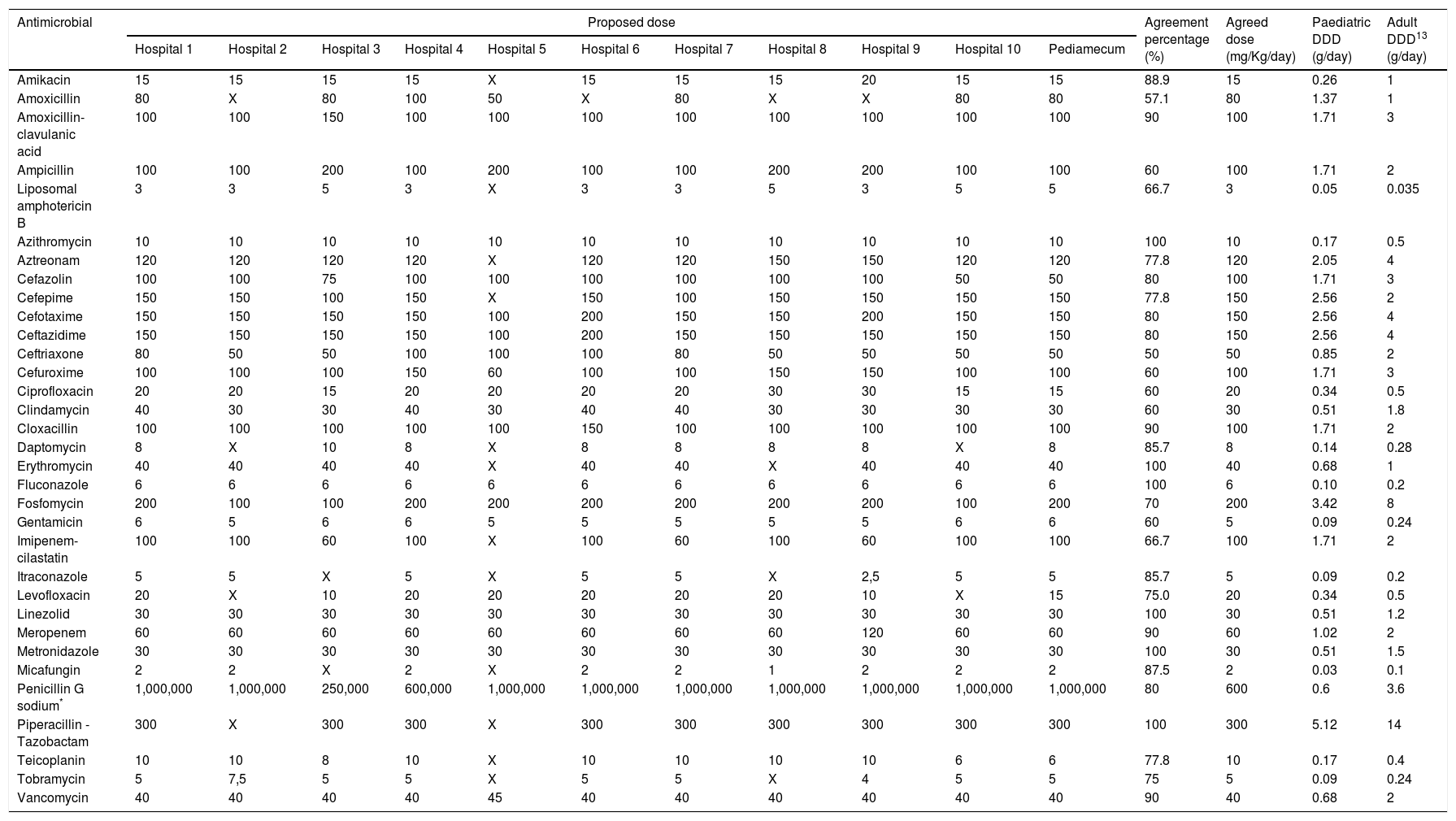
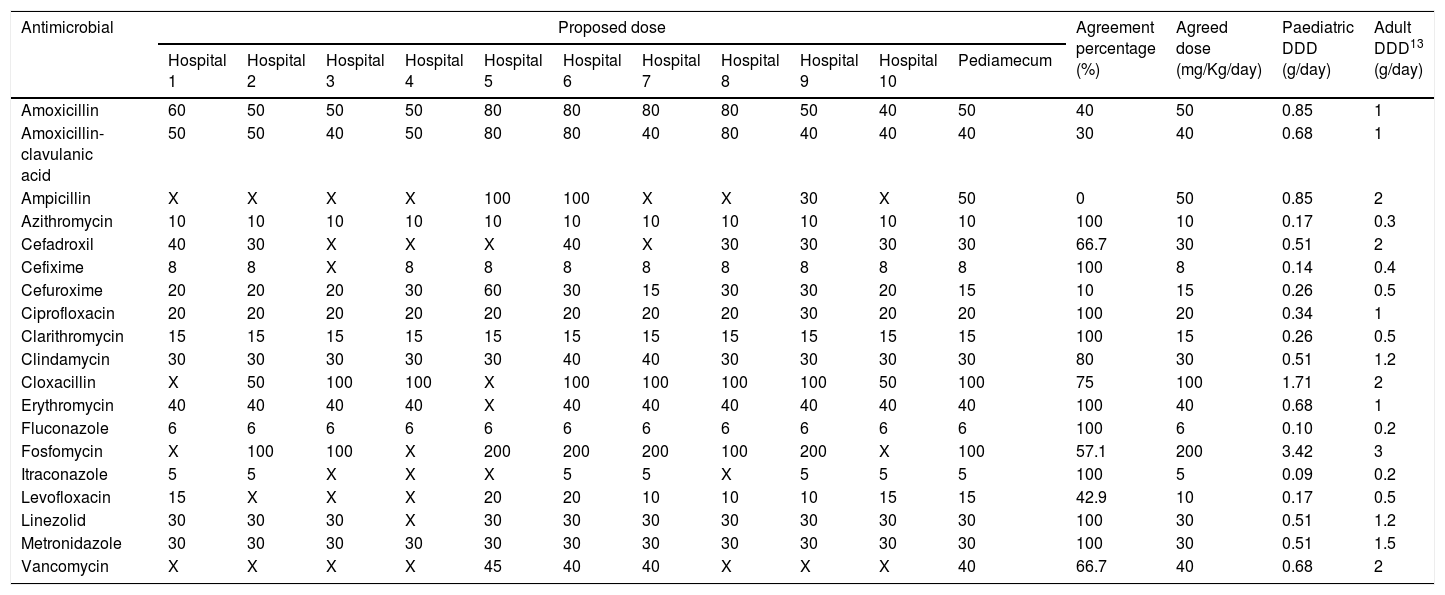
ResultsA total of 40,575 patients were included: 23,877 (58.8%) males, 16,698 (41.2%) females. The median age was 4.17 (IQR: 1.36–8.98) years old for boys and 4.81 (IQR: 1.42–9.60) for girls. The 50th percentile of weight for that age was 17.08kg (males 16.6kg and females 17.8kg). The selected antimicrobials with their respective calculated DDDs and agreement percentages are shown in Table 1 (intravenous route) and Table 2 (oral route). In 9 of 52 antimicrobials, a second round was necessary for dose agreement, achieving it in 4 of them. For oral amoxicillin, amoxicillin-clavulanic acid, ampicillin, cefuroxime and intravenous ceftriaxone; dose was agreed using Pediamecum data. Total agreement percentage was 77.9% (360/462): 79.3% (242/305) and 75.2% (118/157) for intravenous and oral antimicrobials, respectively.
Paediatric defined daily dose of intravenously administered antimicrobials according to the results of Delphi method.
| Antimicrobial | Proposed dose | Agreement percentage (%) | Agreed dose (mg/Kg/day) | Paediatric DDD (g/day) | Adult DDD13 (g/day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4 | Hospital 5 | Hospital 6 | Hospital 7 | Hospital 8 | Hospital 9 | Hospital 10 | Pediamecum | |||||
| Amikacin | 15 | 15 | 15 | 15 | X | 15 | 15 | 15 | 20 | 15 | 15 | 88.9 | 15 | 0.26 | 1 |
| Amoxicillin | 80 | X | 80 | 100 | 50 | X | 80 | X | X | 80 | 80 | 57.1 | 80 | 1.37 | 1 |
| Amoxicillin-clavulanic acid | 100 | 100 | 150 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 90 | 100 | 1.71 | 3 |
| Ampicillin | 100 | 100 | 200 | 100 | 200 | 100 | 100 | 200 | 200 | 100 | 100 | 60 | 100 | 1.71 | 2 |
| Liposomal amphotericin B | 3 | 3 | 5 | 3 | X | 3 | 3 | 5 | 3 | 5 | 5 | 66.7 | 3 | 0.05 | 0.035 |
| Azithromycin | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 100 | 10 | 0.17 | 0.5 |
| Aztreonam | 120 | 120 | 120 | 120 | X | 120 | 120 | 150 | 150 | 120 | 120 | 77.8 | 120 | 2.05 | 4 |
| Cefazolin | 100 | 100 | 75 | 100 | 100 | 100 | 100 | 100 | 100 | 50 | 50 | 80 | 100 | 1.71 | 3 |
| Cefepime | 150 | 150 | 100 | 150 | X | 150 | 100 | 150 | 150 | 150 | 150 | 77.8 | 150 | 2.56 | 2 |
| Cefotaxime | 150 | 150 | 150 | 150 | 100 | 200 | 150 | 150 | 200 | 150 | 150 | 80 | 150 | 2.56 | 4 |
| Ceftazidime | 150 | 150 | 150 | 150 | 100 | 200 | 150 | 150 | 150 | 150 | 150 | 80 | 150 | 2.56 | 4 |
| Ceftriaxone | 80 | 50 | 50 | 100 | 100 | 100 | 80 | 50 | 50 | 50 | 50 | 50 | 50 | 0.85 | 2 |
| Cefuroxime | 100 | 100 | 100 | 150 | 60 | 100 | 100 | 150 | 150 | 100 | 100 | 60 | 100 | 1.71 | 3 |
| Ciprofloxacin | 20 | 20 | 15 | 20 | 20 | 20 | 20 | 30 | 30 | 15 | 15 | 60 | 20 | 0.34 | 0.5 |
| Clindamycin | 40 | 30 | 30 | 40 | 30 | 40 | 40 | 30 | 30 | 30 | 30 | 60 | 30 | 0.51 | 1.8 |
| Cloxacillin | 100 | 100 | 100 | 100 | 100 | 150 | 100 | 100 | 100 | 100 | 100 | 90 | 100 | 1.71 | 2 |
| Daptomycin | 8 | X | 10 | 8 | X | 8 | 8 | 8 | 8 | X | 8 | 85.7 | 8 | 0.14 | 0.28 |
| Erythromycin | 40 | 40 | 40 | 40 | X | 40 | 40 | X | 40 | 40 | 40 | 100 | 40 | 0.68 | 1 |
| Fluconazole | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 100 | 6 | 0.10 | 0.2 |
| Fosfomycin | 200 | 100 | 100 | 200 | 200 | 200 | 200 | 200 | 200 | 100 | 200 | 70 | 200 | 3.42 | 8 |
| Gentamicin | 6 | 5 | 6 | 6 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 60 | 5 | 0.09 | 0.24 |
| Imipenem-cilastatin | 100 | 100 | 60 | 100 | X | 100 | 60 | 100 | 60 | 100 | 100 | 66.7 | 100 | 1.71 | 2 |
| Itraconazole | 5 | 5 | X | 5 | X | 5 | 5 | X | 2,5 | 5 | 5 | 85.7 | 5 | 0.09 | 0.2 |
| Levofloxacin | 20 | X | 10 | 20 | 20 | 20 | 20 | 20 | 10 | X | 15 | 75.0 | 20 | 0.34 | 0.5 |
| Linezolid | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100 | 30 | 0.51 | 1.2 |
| Meropenem | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 120 | 60 | 60 | 90 | 60 | 1.02 | 2 |
| Metronidazole | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100 | 30 | 0.51 | 1.5 |
| Micafungin | 2 | 2 | X | 2 | X | 2 | 2 | 1 | 2 | 2 | 2 | 87.5 | 2 | 0.03 | 0.1 |
| Penicillin G sodium* | 1,000,000 | 1,000,000 | 250,000 | 600,000 | 1,000,000 | 1,000,000 | 1,000,000 | 1,000,000 | 1,000,000 | 1,000,000 | 1,000,000 | 80 | 600 | 0.6 | 3.6 |
| Piperacillin - Tazobactam | 300 | X | 300 | 300 | X | 300 | 300 | 300 | 300 | 300 | 300 | 100 | 300 | 5.12 | 14 |
| Teicoplanin | 10 | 10 | 8 | 10 | X | 10 | 10 | 10 | 10 | 6 | 6 | 77.8 | 10 | 0.17 | 0.4 |
| Tobramycin | 5 | 7,5 | 5 | 5 | X | 5 | 5 | X | 4 | 5 | 5 | 75 | 5 | 0.09 | 0.24 |
| Vancomycin | 40 | 40 | 40 | 40 | 45 | 40 | 40 | 40 | 40 | 40 | 40 | 90 | 40 | 0.68 | 2 |
Abbreviations: DDD: Defined daily dose; X: not used.
Hospital 1: Hospital Universitario Virgen del Rocío, Sevilla; Hospital 2: Hospital Regional Universitario de Málaga; Hospital 3: Complejo Hospitalario Universitario de A Coruña; Hospital 4: Hospital Universitario Cruces, Baracaldo; Hospital 5: Hospital Universitario Infanta Sofía, Madrid; Hospital 6: Complejo Hospitalaria Universitario Insular Materno-Infantil, Las Palmas de Gran Canaria; Hospital 7: Hospital Universitario Reina Sofía, Córdoba; Hospital 8: Hospital Universitario Gregorio Marañón, Madrid; Hospital 9: Hospital Universitario Vall D’Hebrón, Barcelona; Hospital 10: Hospital Universitario de Jerez, Cádiz.
Paediatric defined daily dose of orally administered antimicrobials according to the results of Delphi method.
| Antimicrobial | Proposed dose | Agreement percentage (%) | Agreed dose (mg/Kg/day) | Paediatric DDD (g/day) | Adult DDD13 (g/day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4 | Hospital 5 | Hospital 6 | Hospital 7 | Hospital 8 | Hospital 9 | Hospital 10 | Pediamecum | |||||
| Amoxicillin | 60 | 50 | 50 | 50 | 80 | 80 | 80 | 80 | 50 | 40 | 50 | 40 | 50 | 0.85 | 1 |
| Amoxicillin-clavulanic acid | 50 | 50 | 40 | 50 | 80 | 80 | 40 | 80 | 40 | 40 | 40 | 30 | 40 | 0.68 | 1 |
| Ampicillin | X | X | X | X | 100 | 100 | X | X | 30 | X | 50 | 0 | 50 | 0.85 | 2 |
| Azithromycin | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 100 | 10 | 0.17 | 0.3 |
| Cefadroxil | 40 | 30 | X | X | X | 40 | X | 30 | 30 | 30 | 30 | 66.7 | 30 | 0.51 | 2 |
| Cefixime | 8 | 8 | X | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 100 | 8 | 0.14 | 0.4 |
| Cefuroxime | 20 | 20 | 20 | 30 | 60 | 30 | 15 | 30 | 30 | 20 | 15 | 10 | 15 | 0.26 | 0.5 |
| Ciprofloxacin | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 30 | 20 | 20 | 100 | 20 | 0.34 | 1 |
| Clarithromycin | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 100 | 15 | 0.26 | 0.5 |
| Clindamycin | 30 | 30 | 30 | 30 | 30 | 40 | 40 | 30 | 30 | 30 | 30 | 80 | 30 | 0.51 | 1.2 |
| Cloxacillin | X | 50 | 100 | 100 | X | 100 | 100 | 100 | 100 | 50 | 100 | 75 | 100 | 1.71 | 2 |
| Erythromycin | 40 | 40 | 40 | 40 | X | 40 | 40 | 40 | 40 | 40 | 40 | 100 | 40 | 0.68 | 1 |
| Fluconazole | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 100 | 6 | 0.10 | 0.2 |
| Fosfomycin | X | 100 | 100 | X | 200 | 200 | 200 | 100 | 200 | X | 100 | 57.1 | 200 | 3.42 | 3 |
| Itraconazole | 5 | 5 | X | X | X | 5 | 5 | X | 5 | 5 | 5 | 100 | 5 | 0.09 | 0.2 |
| Levofloxacin | 15 | X | X | X | 20 | 20 | 10 | 10 | 10 | 15 | 15 | 42.9 | 10 | 0.17 | 0.5 |
| Linezolid | 30 | 30 | 30 | X | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100 | 30 | 0.51 | 1.2 |
| Metronidazole | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100 | 30 | 0.51 | 1.5 |
| Vancomycin | X | X | X | X | 45 | 40 | 40 | X | X | X | 40 | 66.7 | 40 | 0.68 | 2 |
Abbreviations: DDD: Defined daily dose; X: not used.
Hospital 1: Hospital Universitario Virgen del Rocío, Sevilla; Hospital 2: Hospital Regional Universitario de Málaga; Hospital 3: Complejo Hospitalario Universitario de A Coruña; Hospital 4: Hospital Universitario Cruces, Baracaldo; Hospital 5: Hospital Universitario Infanta Sofía, Madrid; Hospital 6: Complejo Hospitalaria Universitario Insular Materno-Infantil, Las Palmas de Gran Canaria; Hospital 7: Hospital Universitario Reina Sofía, Córdoba; Hospital 8: Hospital Universitario Gregorio Marañón, Madrid; Hospital 9: Hospital Universitario Vall D’Hebrón, Barcelona; Hospital 10: Hospital Universitario de Jerez, Cádiz.
Paediatric DDDs for antimicrobials have been designed in this multicentre study. The best metric for aggregate antimicrobial consumption evaluation in paediatrics has not yet being defined.4,10 DDD measurement in children is limited due to weight variability, thus, other methods like Point Prevalence Surveys (PPS) have been proposed.2,11,12 PPS can assess antimicrobial consumption in short periods of time basis, using retrospective, prospective or mixed designs.10,12 The Antibiotic Resistance and Prescribing in European Children Project (ARPEC) and the European Surveillance of Antimicrobial Consumption (ESAC), have used this metric to monitor antimicrobial consumption in children.10,11 However, PPS specific time-point data is susceptible to case-mix complexity, seasonality and variability of the sample.
Current recommendation for paediatric ASP metric is Days of Therapy (DOT).5 This metric counts the number of days a patient receives antibiotics, avoiding weight variability. Nonetheless, DOT measurement poses problems in the setting of drugs with a long half-life, variable pharmacodynamics, or in patients with renal failure.5 In addition, DOT favours patients who receive less antimicrobials even if that involves the use of broader spectrum antimicrobials,5 while the optimal length of treatment for most of paediatric infections varies widely.12
Few studies have used the DDD methodology in children.4 A recent systematic review found up to 26 distinct measures across 79 studies including paediatric patients (DDD or similar metrics in 38 studies).4 Limitations were found for all metrics and authors conclude that the best metric remains to be identified.4 In our study, weight variability was overcome by selecting the average weight for the obtained sample. The availability of a normalized DDD value could allow its application for ASP monitoring in paediatrics. Nevertheless, the average weight obtained in our sample, does not allow comparisons with other series that may report a different median age and consequently a different average weight. Future strategies could include constructing confidence intervals for the calculated DDDs or designing specific DDDs for paediatric age subgroups of interest (neonates, infants, children, adolescents).
Centre participation was high for the selected dose agreement method, therefore, the lack of dose values for some antimicrobials does not reflect data loss, it could be due to the variability of antimicrobial use in the included centres. Thus, it is possible that some agreed DDDs of occasionally used drugs are less reliable, as well as for those antimicrobials for which there were more discrepancies between centres. Nevertheless, dose agreement data was high for most of the antimicrobials included, and further confirmation would be expected when this tool undergoes validation.
Of note, 4 out of 52 antimicrobials had a greater paediatric DDD when compared to adult DDD. For amoxicillin and liposomal amphotericin B, the DDD value established by WHO is smaller than the dose used in clinical practice according to the specific antimicrobial drug label instructions.13 For cefepime and oral fosfomycin the agreed doses where greater in paediatrics probably because these antimicrobials are used mostly for infections that require higher doses in children.14
Main limitations of our study include the lack of validation of this strategy and low external validity of the method. To overcome this, a new multicentre study has been projected to validate this tool in real-life patients who receives antimicrobial prescriptions under usual clinical practice. Currently, the project is looking for internal validity in our country including representative centres of several Spanish regions. If good results are obtained, further international multi-centre studies will be proposed to standardize this metric.5,6 Although standardized Spanish weigh for age and sex charts exist,15 WHO charts were used with the aim to provide a tool potentially applicable in different populations.
To the best of our knowledge, this study provides one of the largest sample size to define specific DDD for paediatric population, in a multicentre setting. Neonatal patients were excluded, as they have different pharmacokinetics and pharmacodynamics compared to paediatric patients. A parallel project is underway to design neonatal DDD applying a similar methodology.
In conclusion, this study has designed a suitable paediatric DDD for antimicrobial consumption assessment. Further validation of this tool standardizing the age ranges is required in future studies, as this metric could help to design paediatric ASP strategies to prevent the emergence and dissemination of antimicrobial resistance in children.
FundingNo specific funding was received for this article.
Conflicts of interestThe authors declare no conflicts of interest.
Collaborator-investigators: Concepción Álvarez-Vayo Benito, Department of Pharmacy, Institute of Biomedicine of Seville (IBIS), Hospital Universitario Virgen del Rocío, Sevilla, Spain; Estíbaliz Chavarri-Gil, Department of Pharmacy, Hospital Universitario de Cruces, Bilbao, Spain; Carmen Gallego-Fernández, Department of Pharmacy, Hospital Universitario de Málaga, Málaga, Spain; David Moreno-Pérez, Paediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitario de Málaga, IBIMA Research Group, Department of Paediatrics and Pharmacology, Faculty of Medicine of the University of Málaga, Málaga, Spain; Elisenda Dolz, Department of Pharmacy, Complejo Hospitalario Universitario Materno-Infantil, Gran Canaria, Spain; Alfredo Tagarro-García, Department of Paediatrics, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, Madrid, Spain; José Rumbao-Aguirre, Department of Paediatrics, Critical Care and Emergencies, Hospital Universitario Reina Sofía, Facultad de Medicina, Córdoba, Spain; José-María Gutiérrez-Urbón, Department of Pharmacy, Complejo Hospitalario Universitario A Coruña, Spain.