Community Acquired Pneumonia (CAP) is common disease that can be treated in Hospital At Home (HAH). In this paper we evaluate the room of improvement in the use of antibiotics in CAP in HH.
MethodsPatients with CAP were retrospectively recruited in two Spanish hospitals from 1/1/18 to 10/30/19. Demographic, clinical and quality of antibiotic prescription variables were recorded. Subsequently, we created a new variable that collected six quality of care indicator, categorizing and comparing patients into two groups: good quality of care (4 or more indicators performed) or poor quality of care (3 or less indicators performed).
ResultsWe recruited 260 patients. The request for diagnostic tests and the adequacy to Clinical Practice Guidelines were 85.4% and 85.8% respectively. Percentages of de-escalation (53.7%) and sequential therapy (57.7%) when indicated were low. The average length of treatment was 7.3 days for intravenous and 9.5 days for total. Quality of prescription was good in 134 (63.2%) patients, being more frequent in those who were admitted directly to HAD from the emergency room. It was also associated with less readmission at 30 days.
ConclusionThere is a wide room for improvement in some fields of antimicrobials use in HAH that could stimulate the implementation of Antimicrobial Stewardship Programs.
La Neumonía Adquirida en la Comunidad (NAC) es una enfermedad frecuente que puede ser abordada en Hospitalización a Domicilio (HAD). En el presente trabajo evaluamos el margen de mejora en el uso de antibióticos en la NAC en HAD.
MétodosSe reclutaron retrospectivamente todos los pacientes con NAC en dos hospitales españoles desde el 01 de enero de 2018 al 30 de octubre de 2019. Se registraron variables demográficas, clínicas y sobre calidad de prescripción antibiótica. Posteriormente se construyó una variable que recogía seis indicadores de calidad asistencial, categorizando y comparando a los pacientes en dos grupos: buena calidad asistencial (cuatro o más indicadores realizados) o mala calidad asistencial (tres o menos indicadores realizados).
ResultadosObtuvimos una muestra de 260 pacientes. La solicitud de pruebas diagnósticas y la adecuación a las guías de práctica clínica fue del 85,4 y 85,8%, respectivamente. Los porcentajes de realización de desescalada (53,7%) y terapia secuencial (57,7%) cuando estaban indicadas fueron bajos. La duración media del tratamiento fue de 7,3 días para el intravenoso y 9,5 días para el total. La calidad de prescripción fue buena en 134 (63,2%) pacientes, siendo más frecuente en aquellos que ingresaron directamente en HAD desde urgencias. También se asoció a menor reingreso a 30 días.
ConclusiónExiste un amplio margen de mejora en algunos aspectos con el uso de antimicrobianos en HAD, que podría motivar la implementación de programas de optimización del uso de antibióticos.
The healthcare burden associated with community-acquired pneumonia (CAP) in Spain is high, with an incidence and hospitalisation rate of 4.63 and 1.64 cases per 1000 people/year respectively.1,2 Hospital at home (HaH) has been established as a useful and safe option for treating these patients, taking into account that the antibiotic management should be no different from that in CAP with conventional hospitalisation.3
In the management of CAP in HaH, the rational use of antibiotics has the same validity as in conventional hospitalisation. There are various indicators associated with improvement in the treatment of CAP, such as adherence to the clinical practice guidelines (CPG) in terms of treatment,4 de-escalation (reduction of the spectrum and ecological impact of the antimicrobial when indicated),5 sequential therapy (switch to oral route after intravenous treatment)6 and the appropriate duration of treatment (five to seven days, in uncomplicated cases).7,8
In view of the limited amount of literature on this subject, we decided to assess the quality of antibiotic prescribing in patients with CAP in HaH at two Spanish hospitals, Hospital Reina Sofía (HRS) in Tudela and Hospital Royo Villanova (HRV) in Zaragoza, with the aim of determining how much room there was for improvement.
MethodsAll cases of CAP under the HaH regimen in patients over 18 years of age from 1 January 2018 to 30 October 2019 were collected retrospectively. CAP was considered based on two assumptions: if the patient’s doctor diagnosed them with CAP and recorded it as such in their medical records; or if the patient had typical symptoms and radiological consolidation. Bronchoaspiration and pneumonia in institutionalised patients were included. Nosocomial pneumonia and respiratory infection without consolidation were excluded.
We recorded demographic (age, comorbidities or Charlson index), clinical (epidemiological context or severity) and outcome variables. We determined the quality of antibiotic prescribing during the hospitalisation, both on the ward and at home, using five variables. The first was the request for microbiology tests. The second, adherence to the CPG (local if applicable or if not general) in terms of the choice of antibiotic. The third, whether or not de-escalation was indicated and carried out, for which we considered the microbiological results, the clinical progress of the patient and the likelihood of resistant bacteria, understood as the likely presence of methicillin-resistant S. aureus (MRSA) if there had been previous isolation, or of P. aeruginosa if there had been previous isolation, or two of the following criteria were met: severe COPD with FEV1 <30%; presence of generalised bronchiectasis; recent hospitalisation; recent administration of antibiotics (three months) or more than four courses of antibiotics in the last year; and taking an oral corticosteroid, more than 10 mg of prednisolone or similar, in the last two weeks. The fourth was whether or not sequential therapy was indicated and given, for which we considered whether the patient tolerated the oral route and had become clinically stable, with that point defined as when the patient had managed a 24-h period with their temperature below 38 °C, systolic blood pressure above 90 mmHg, heart rate less than 100 beats per minute, respiratory rate less than 24 respirations per minute and arterial oxygen saturation greater than 90% with, at most, oxygen via nasal cannula. The fifth was the duration of the treatment. Finally, a dichotomous variable was constructed to assess the prescribing quality. One point was awarded to each of the following indicators: request for a microbiological test, adherence to the CPG in the choice of drug; de-escalation carried out or not indicated; sequential therapy carried out or not indicated; duration of intravenous treatment less than five days; and total treatment duration less than seven days. Next, the patients for whom the antibiotic prescribing was good quality (four to six indicators applied) were compared to those where it was not (three or fewer indicators applied). For this analysis, we excluded cases where the patient was admitted directly to hospital at home without passing through Accident and Emergency, cases where the patient died during the admission, and those with reasons for prolonging the treatment (complications such as empyema, abscess or bacterial superinfection and infection by P. aeruginosa and MRSA), thereby trying to select patients with uncomplicated CAP. Lastly, for the outcome variables, we recorded the length of stay and the death and readmission rates, both during hospitalisation and within 30 days after discharge. A check was made for any medical consultations or outpatient tests to confirm that the patients were still alive at some point after discharge. A team of doctors collected the data by reviewing medical records and interpreted the suitability of the indicators.
For the descriptive analysis, percentages, means and quartiles were used. For hypothesis testing, we used the χ2 test, Fisher’s exact test and the odds ratio (OR) for qualitative variables and the Mann–Whitney U test for quantitative variables (variables not showing normal distribution). A p-value of <0.05 was considered statistically significant. We used the SPSS® software version 15. Authorization was obtained from the Navarra Independent Ethics Committee.
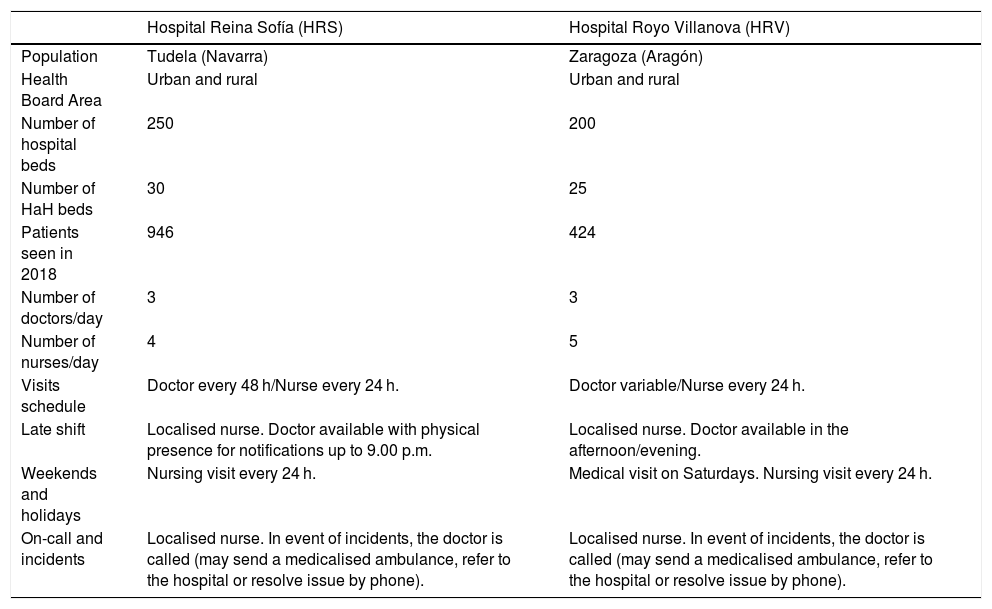
ResultsCharacteristics of the hospitals and general descriptionThe study was carried out at the hospital centres HRS and HRV. The characteristics of these hospitals and their respective HaH services are shown in Table 1. A total of 260 patients were recruited whose main variables are listed in Table 2.
Characteristics of the two hospitals in the study.
| Hospital Reina Sofía (HRS) | Hospital Royo Villanova (HRV) | |
|---|---|---|
| Population | Tudela (Navarra) | Zaragoza (Aragón) |
| Health Board Area | Urban and rural | Urban and rural |
| Number of hospital beds | 250 | 200 |
| Number of HaH beds | 30 | 25 |
| Patients seen in 2018 | 946 | 424 |
| Number of doctors/day | 3 | 3 |
| Number of nurses/day | 4 | 5 |
| Visits schedule | Doctor every 48 h/Nurse every 24 h. | Doctor variable/Nurse every 24 h. |
| Late shift | Localised nurse. Doctor available with physical presence for notifications up to 9.00 p.m. | Localised nurse. Doctor available in the afternoon/evening. |
| Weekends and holidays | Nursing visit every 24 h. | Medical visit on Saturdays. Nursing visit every 24 h. |
| On-call and incidents | Localised nurse. In event of incidents, the doctor is called (may send a medicalised ambulance, refer to the hospital or resolve issue by phone). | Localised nurse. In event of incidents, the doctor is called (may send a medicalised ambulance, refer to the hospital or resolve issue by phone). |
HaH: hospital at home.
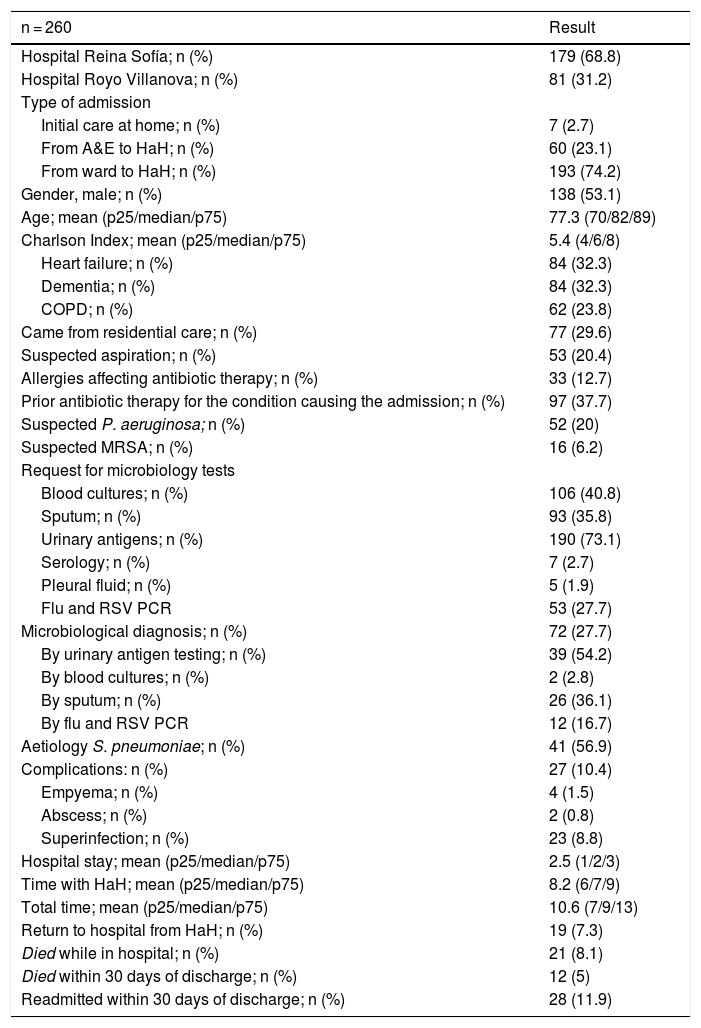
Demographic and clinical characteristics and results for the sample.
| n = 260 | Result |
|---|---|
| Hospital Reina Sofía; n (%) | 179 (68.8) |
| Hospital Royo Villanova; n (%) | 81 (31.2) |
| Type of admission | |
| Initial care at home; n (%) | 7 (2.7) |
| From A&E to HaH; n (%) | 60 (23.1) |
| From ward to HaH; n (%) | 193 (74.2) |
| Gender, male; n (%) | 138 (53.1) |
| Age; mean (p25/median/p75) | 77.3 (70/82/89) |
| Charlson Index; mean (p25/median/p75) | 5.4 (4/6/8) |
| Heart failure; n (%) | 84 (32.3) |
| Dementia; n (%) | 84 (32.3) |
| COPD; n (%) | 62 (23.8) |
| Came from residential care; n (%) | 77 (29.6) |
| Suspected aspiration; n (%) | 53 (20.4) |
| Allergies affecting antibiotic therapy; n (%) | 33 (12.7) |
| Prior antibiotic therapy for the condition causing the admission; n (%) | 97 (37.7) |
| Suspected P. aeruginosa; n (%) | 52 (20) |
| Suspected MRSA; n (%) | 16 (6.2) |
| Request for microbiology tests | |
| Blood cultures; n (%) | 106 (40.8) |
| Sputum; n (%) | 93 (35.8) |
| Urinary antigens; n (%) | 190 (73.1) |
| Serology; n (%) | 7 (2.7) |
| Pleural fluid; n (%) | 5 (1.9) |
| Flu and RSV PCR | 53 (27.7) |
| Microbiological diagnosis; n (%) | 72 (27.7) |
| By urinary antigen testing; n (%) | 39 (54.2) |
| By blood cultures; n (%) | 2 (2.8) |
| By sputum; n (%) | 26 (36.1) |
| By flu and RSV PCR | 12 (16.7) |
| Aetiology S. pneumoniae; n (%) | 41 (56.9) |
| Complications: n (%) | 27 (10.4) |
| Empyema; n (%) | 4 (1.5) |
| Abscess; n (%) | 2 (0.8) |
| Superinfection; n (%) | 23 (8.8) |
| Hospital stay; mean (p25/median/p75) | 2.5 (1/2/3) |
| Time with HaH; mean (p25/median/p75) | 8.2 (6/7/9) |
| Total time; mean (p25/median/p75) | 10.6 (7/9/13) |
| Return to hospital from HaH; n (%) | 19 (7.3) |
| Died while in hospital; n (%) | 21 (8.1) |
| Died within 30 days of discharge; n (%) | 12 (5) |
| Readmitted within 30 days of discharge; n (%) | 28 (11.9) |
A&E: Accident and Emergency department; HaH: hospital at home; p25; median; p75: quartiles and median; COPD: chronic obstructive pulmonary disease; MRSA: methicillin-resistant Staphylococcus aureus; PCR: polymerase chain reaction; RSV: respiratory syncytial virus.
Some microbiological test was requested in 222 (85.4%) patients, with a microbiological diagnosis being made in 72 (32.4% of those attempted) (Table 2).
Adherence to CPGThe choice of antibiotic was suitable in 223 (85.8%) patients. The choice consisted of ceftriaxone monotherapy in 91 (35%) cases, levofloxacin monotherapy in 50 (19.2%), and ceftriaxone plus azithromycin, clarithromycin or levofloxacin in 57 (21.9%). Thirty-nine (15%) started treatment with carbapenems or piperacillin/tazobactam.
De-escalationDe-escalation was indicated in 41 (15.8%) patients, and carried out in 22 of them (53.7%). Of the 41 with the indication, microbiological isolation was achieved in 25 (61%). Out of the 22 who had de-escalation, in 16 (72%) it was carried out within 24 h of being indicated.
Sequential therapySequential therapy was potentially possible in 201 (77.3%) patients, and was used in 116 (57.7%). Two hundred and forty-six (94.6%) achieved clinical stability and oral tolerance at some point during admission. Of these patients, 115 had sequential therapy. The time to the switch to oral was 3.9 days on average (p25, 2; median, 4; p75, 5).
Duration of antibiotic therapyExcluding 21 (9.1%) patients who died while admitted and 26 (10%) who had complications, P. aeruginosa or MRSA, the duration of the antibiotic therapy was analysed in 213 (81.9%). The mean duration of intravenous therapy was 7.3 days (p25, 5; median, 7; p75, 9), and mean total duration of the therapy was 9.5 days (p25, 7; median, 9; p75, 11). In the patients excluded for complications, P. aeruginosa or MRSA (26), the mean duration of intravenous therapy was 10.8 days and the mean total duration of therapy was 15.3 days.
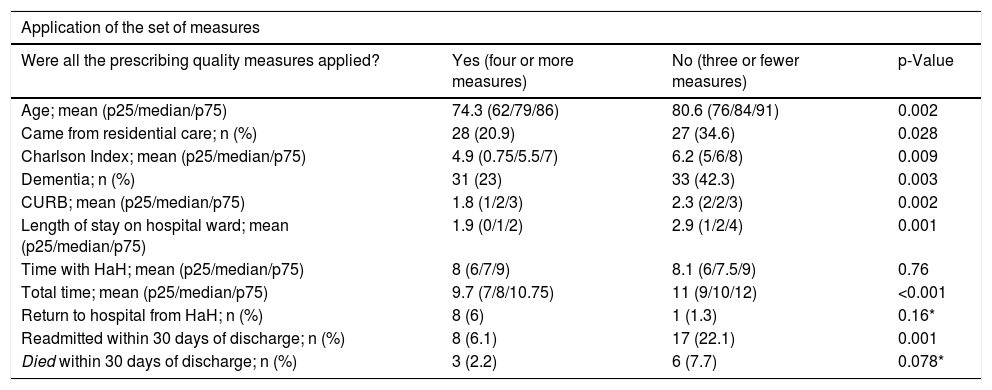
Comparison based on the quality of antibiotic prescribingWe were able to apply the analysis of the set of prescribing quality indicators in 212 (81.5%) patients. In 134 (63.2%) prescribing quality was good (four or more quality indicators), these patients being younger and with fewer comorbidities, less severity and shorter length of stay. There were differences between hospitals. HRV had good quality prescribing in 82% of the patients compared to 55.6% at HRS (p < 0.001). The prescribing quality was better in patients transferred from the Accident and Emergency to HaH, than in those who spent time on the ward; (75.9% good quality vs 59.5%, p = 0.031; OR 0.46 (95% CI 0.23–0.94). It was also associated with not being readmitted within 30 days; OR 0.26 (95% CI 0.11–0.59). There were no significant differences in the rest of the variables (Table 3).
Example of data interpretation (31 patients [23%] who had good quality prescribing [four or more measures] had dementia, compared to 33 [42.3%] of the group that did not have good quality prescribing having dementia; dementia was more common in the group that did not have good quality prescribing, with p = 0.003).
| Application of the set of measures | |||
|---|---|---|---|
| Were all the prescribing quality measures applied? | Yes (four or more measures) | No (three or fewer measures) | p-Value |
| Age; mean (p25/median/p75) | 74.3 (62/79/86) | 80.6 (76/84/91) | 0.002 |
| Came from residential care; n (%) | 28 (20.9) | 27 (34.6) | 0.028 |
| Charlson Index; mean (p25/median/p75) | 4.9 (0.75/5.5/7) | 6.2 (5/6/8) | 0.009 |
| Dementia; n (%) | 31 (23) | 33 (42.3) | 0.003 |
| CURB; mean (p25/median/p75) | 1.8 (1/2/3) | 2.3 (2/2/3) | 0.002 |
| Length of stay on hospital ward; mean (p25/median/p75) | 1.9 (0/1/2) | 2.9 (1/2/4) | 0.001 |
| Time with HaH; mean (p25/median/p75) | 8 (6/7/9) | 8.1 (6/7.5/9) | 0.76 |
| Total time; mean (p25/median/p75) | 9.7 (7/8/10.75) | 11 (9/10/12) | <0.001 |
| Return to hospital from HaH; n (%) | 8 (6) | 1 (1.3) | 0.16* |
| Readmitted within 30 days of discharge; n (%) | 8 (6.1) | 17 (22.1) | 0.001 |
| Died within 30 days of discharge; n (%) | 3 (2.2) | 6 (7.7) | 0.078* |
Asterisk (*), Fisher’s correction applied.
HaH: hospital at home; p25, p50, p75: quartiles and median; CURB: pneumonia prognostic scale (confusion, urea, respiratory rate, blood pressure).
We found substantial room for improvement in the quality of antibiotic prescribing in our sample. Although microbiological tests were largely requested and the choice of treatment did adhere to CPG,7,9 we found that almost half of the patients could have benefited from de-escalation and sequential therapy (46.3% and 42.3% respectively). The duration of treatment could also have been optimised (7.3 days of intravenous treatment and 9.5 days in total).
De-escalation was carried out in very few patients. A number of studies have shown de-escalation to be safe in CAP, even with bacteraemia.5,10,11 De-escalating would be more complex in patients without microbiological isolation (in our sample, 39%). Nevertheless, we believe that in these patients a broader-spectrum antibiotic than necessary was chosen and that within a few days, clinical situation permitting, it could have been reduced. Despite this, it only took a day to carry out de-escalation when it was indicated, which is positive. As regards sequential therapy, we think that this may be the most important measure to be implemented due to its simplicity and the margin for improvement. There is sufficient evidence in CAP in conventional hospitalisation to support the fact that switching to the oral route once the patient is clinically stable is safe, even in severe CAP, reducing complications and length of stay.6,12 In a Dutch study on CAP in conventional hospitalisation, rates similar to ours were detected (although sequential therapy was indicated in 46% of patients, the switch was not made in 40% of them), with misconceptions, practical considerations and organisational factors identified as barriers to the proper application of said measure.13 Furthermore, in our sample it took 3.9 days on average to switch to oral therapy, which is too long. Last of all, we found room for improvement in the duration of antibiotic treatment in uncomplicated CAP. The optimal duration established in other studies7,8 would be in the 25th percentile in our sample (five days intravenous, seven days in total), not omitting the fact that one in four patients was treated for nine or more days with intravenous and 11 or more days in total. Improving these figures would reduce length of stay, complications and antibiotic use.
Our study has a number of limitations. Firstly, given the observational nature, the investigators who reviewed the medical records may have missed elements being considered at the time by the physician which might have led them to depart from the CPG advice. There is also a series of interpretation biases due to the fact that most of the studies on CAP have been carried out in conventional hospitalisation, raising the question of whether they would be comparable in HaH. With regard to sequential therapy, it is important to take into account that eligibility for HaH may be due to compromise of the oral route or the patient requiring intravenous antimicrobial therapy at home (Outpatient Parenteral Antibiotic Therapy [OPAT] at home), although we believe we have minimised this bias with the variables oral tolerance and stability. Also, the working dynamics of HaH could have caused the loss of days and the delay in the implementation of measures, due to the fact that the medical visits are only every 48 h and that a certain amount of clinical inertia may prevail, even when the clinical and microbiological data offer new information. These assumptions make our study all the more relevant, as not only have we shone a light on a subject area where there is little available literature, but we have identified a number of priority elements for implementing improvements in antibiotic prescribing in HaH. Last of all, our interpretation is that the differences between the group with good-quality prescribing (four to six indicators) and the group with poor-quality prescribing (three or fewer indicators) could be due to the fact that the poor-quality group had older patients with more comorbidities. One explanation for the prescribing being worse in these patients could be frailty and comorbidities leading to underestimating of the clinical improvement in the infectious aspects of CAP, delaying the implementation of these quality indicators. Although we consider that the lower risk of readmission at 30 days in the group with good quality of care (OR 0.26) may have been due to the baseline characteristics of these patients (younger and fewer comorbidities), we believe that the differences found between those admitted directly to HaH from Accident and Emergency and those admitted to HaH after spending one or several days on a conventional ward (the prescribing quality was better in the first group with an OR 0.46), could actually be related to the care dynamics in HaH, which would point to another area for improvement. It is possible that having the same doctor assess a patient from beginning to end (admission to HaH for the entire course of the disease) may make it easier to apply the indicators that depend on the patient’s progress (de-escalation, sequential therapy and duration), compared to cases in which several doctors or systems (conventional ward and HaH) have treated these patients. This assumption could be optimised by improving communication between the hospital ward and HaH doctors or by planning indicators from day zero of care.
The optimisation of antimicrobial prescribing is a topic of growing interest due to its implications for patient health, efficiency of healthcare systems and the emergence of bacterial resistance. These measures need to be applied in all aspects of medicine and HaH is one area which has been little studied to date. We found significant room for improvement in the quality of antimicrobial use in CAP in HaH, which could also translate to other infectious diseases. We consider that this would be an interesting area for antimicrobial stewardship programmes (ASP) to address.14
FundingNo funding was received for this study.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez Fabra D, Ger Buil A, Torres Courchoud I, Martínez Murgui R, Matía Sanz MT, Fiteni Mera I, et al. Manejo antibiótico en neumonía adquirida en la comunidad en la hospitalización a domicilio: ¿hay margen de mejora? Enferm Infecc Microbiol Clin. 2021;39:271–275.