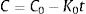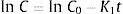Prolonged intravenous infusion of beta-lactams increase the clinical cure rate compared to conventional administration in critical or septic patients. This study aimed to determine chemical stability and physical compatibility of meropenem at conditions used in clinical practice to evaluate the stability of the preparation during its administration and the possibility of anticipated preparation.
MethodsAdmixtures in study were: (i) meropenem 6g in 0.9% sodium chloride (NS) in infusor of 2mL/h 50mL or 10mL/h 240mL; (ii) meropenem 1 or 2g in NS in infusion bag of 250mL. Temperatures of study were: (i) infusor: 4.5°C, 32°C or 12h at 4.5°C followed by 32°C; (ii) Infusion bag: 4.5°C, 24.5°C or 6h at 4.5°C followed by 24.5°C. Time of study was 5–6 days in infusor and 1 day in infusion bag. Chemical stability was evaluated by high performance liquid chromatography and physical compatibility by measuring pH and visual inspection.
ResultsChemical stability and physical compatibility of meropenem in admixtures in infusors were reduced at high meropenem concentration and high temperature. Admixtures in infusion bag show chemical stability and physical compatibility for at least 1 day.
ConclusionAdministration of meropenem 6g in infusion of 24h in 240mL of 0.9% NaCl in infusor of 10mL/h could be possible if the admixture is infused at 4.5°C. Extended infusion of meropenem 1 or 2g in 0.9% NaCl in infusion bag (250mL) in 3–4h is also feasible. Anticipated preparation of the admixtures in infusion bag is possible with a stability of 24h.
La infusión intravenosa prolongada de beta-lactámicos aumenta la velocidad de curación clínica comparada con la administración convencional en pacientes críticos o sépticos. Este estudio tiene como objetivo determinar la estabilidad química y la compatibilidad física de meropenem en condiciones utilizadas en la práctica clínica para evaluar la estabilidad de la preparación durante su administración y la posibilidad de la preparación anticipada.
MétodosLas mezclas en estudio fueron: (I) meropenem 6g en cloruro sódico 0,9% (SN) en infusor de 2mL/h 50 mL o 10mL/h 240mL; (iii) meropenem 1 o 2g en SN en bolsa de infusión de 250mL. Las temperaturas de estudio fueron: (i) infusor: 4,5°C, 32°C o 12h a 4,5°c seguido de 32°C; (ii) bolsa de infusión: 4,5°C, 24,5°C o 6h a 4,5°c seguido de 24,5°C. El tiempo de estudio fue de 5-6 días en infusor y 1 día en bolsa de infusión. Se evaluó la estabilidad química mediante cromatografía líquida de alta resolución y la compatibilidad física por medida de pH e inspección visual.
ResultadosLa estabilidad química y la compatibilidad física de meropenem en las mezclas en infusores disminuyeron al aumentar la concentración de meropenem y la temperatura. Las mezclas en bolsas de infusión mostraron estabilidad química y compatibilidad física durante al menos 1 día.
ConclusiónLa administración de meropenem 6g en infusión de 24h en 240 mL de cloruro sódico 0,9% en infusor de 10ml/h podría ser posible si la mezcla es administrada a 4,5°C. La infusión extendida de 1 o 2g en cloruro sódico 0,9% en bolsa de infusión (250 mL) en 3-4h es también viable. Puede realizarse la preparación anticipada de mezclas de meropenem en bolsas de infusión con una estabilidad de 1 día.
Meropenem is a broad-spectrum antibiotic with bactericidal activity against Gram positive and negative bacteria. Meropenem acts by inhibiting the synthesis of the bacterial cell wall, and it is indicated in the treatment of serious lung, urinary, intrabdominal, skin and soft tissue infections or meningitis, with dosage ranging from 1g every 8h to 2g in lung infections associated with cystic fibrosis or acute bacterial meningitis.1
The efficacy of meropenem is correlated with the time that plasma concentrations exceed the minimum inhibitory concentration of the pathogen (T>MIC).2 In carbapenems, the PK-PD objective is to achieve T>MIC during approximately 40% of the dosing interval,1 though this objective has not been established in the clinical setting and studies in critically ill patients point to better clinical and microbiological results with 100% T>MIC.3,4 The carbapenem MIC breakpoint, according to the international reference organizations, will determine the sensitivity or resistance of the microorganism to the antibacterial. In order to achieve clinical cure of serious infections and prevent antimicrobial resistance, dose optimization is needed.5
Meropenem 1g is usually administered by intermittent infusion (II) over approximately 30min, producing a peak plasma concentration of 50–60mg/L, which drops to 0.25mg/L after 8h.1 Several studies evidence that prolonged infusions of meropenem, including extended infusion (EI) and continuous infusion (CI), provide greater likelihood of reaching the T>MIC target than standard dosage forms.6–9 CI would ideally achieve a higher T>MIC, but it has drawbacks such as permanent attachment to the line, Y-site incompatibilities, and mainly stability problems associated with carbapenems.10 However, EI of 1 or 2g of meropenem each 8h stands as an effective alternative since it increases the T>MIC, avoiding the disadvantages associated with the CI. Jaruratanasirikul et al.11 point out that EI would achieve the PK-PD objective of 40% T>MIC with a likelihood of 90% compared to 79% with II for doses of 0.5g/8h (MIC≤4mg/L), 1g/8h (MIC≤8mg/L) and 2g/8h (MIC≤16mg/L).
To ensure that patients receive sufficient meropenem to achieve cure while avoiding exposure to toxic degradation products, chemical stability and physical compatibility of meropenem should be maintained throughout the period of administration. Stability data available until now indicate that meropenem in 0.9% sodium chloride (NS) at concentrations ranged from 4 to 20mg/mL is stable for 3–7 days at 5°C in elastomeric infusion devices for CI12,13 and that meropenem 1 or 2g in 100mL with NS (10 and 20mg/mL, respectively) is stable for 5 days at 5°C and 10h at 25°C in infusion bags for EI.12–14
The objective of the present study is to determine the chemical stability and physical compatibility of meropenem in elastomeric infusion devices and infusion bags at concentrations used in clinical practice (25 and 120mg/mL in infusors; 4 and 8mg/mL in infusion bags) at different temperatures (4.5, 24.5 or 32°C), in order to evaluate the stability of the preparation during its administration by CI or EI and the possibility of anticipated preparation of admixtures.
MethodsReagents and materialsAs reagents, meropenem 1000mg powder for injection (Aurovitas Spain, Barcelona, Spain), NS in Viaflo® infusion bag (made of polyethylene, polyamide and polypropilen) of 50 or 250mL (Baxter, Barcelona, Spain), sterile water (API) for injection (B. Braun, Barcelona, Spain), acetonitrile (Scharlab, Barcelona, Spain) and sodium dihydrogen phosphate (NaH2PO4; Panreac, Barcelona, Spain) were adquired.
Singleday® 2mL/h 50mL and Folfusor® LV 10mL/h 240mL (Baxter, Barcelona, Spain) elastomeric infusion devices (made of polyisoprene) and light protection bags (Diffuplast, Italy) were used.
AdmixturesAdmixtures were prepared by duplicate following Good Manufacturing Practice (GMP)15, being 24 the total number of admixtures. Table 1 shows dose, volume and container of the admixtures. To prepare them, meropenem powder was reconstituted with API and dilluted to the corresponding volume with NS. All admixtures were introduced in ligh protection bags.
Dose, volume, container, temperature and time of the study of the admixtures of meropenem.
| AD | Dose (g) | V (mL) | Container | T (°C) | t (days) |
|---|---|---|---|---|---|
| 1–2 | 6 | 50 | Singleday® 2mL/h 50mL | 4.5 | 5 |
| 3–4 | 6 | 50 | Singleday® 2mL/h 50mL | 32 | 5 |
| 5–6 | 6 | 50 | Singleday® 2mL/h 50mL | 12h at 4.5°C+32°C | 6 |
| 7–8 | 6 | 240 | Folfusor® LV 10mL/h 240mL | 4.5 | 5 |
| 9–10 | 6 | 240 | Folfusor® LV 10mL/h 240mL | 32 | 5 |
| 11–12 | 6 | 240 | Folfusor® LV 10mL/h 240mL | 12h at 4.5°C+32°C | 6 |
| 13–14 | 1 | 250 | Viaflo® | 4.5 | 1 |
| 15–16 | 1 | 250 | Viaflo® | 24.5 | 1 |
| 17–18 | 1 | 250 | Viaflo® | 6h at 4.5°C+24.5°C | 1 |
| 19–20 | 2 | 250 | Viaflo® | 4.5 | 1 |
| 21–22 | 2 | 250 | Viaflo® | 24.5 | 1 |
| 23–24 | 2 | 250 | Viaflo® | 6h at 4.5°C+24.5°C | 1 |
AD: admixture; V: final volume; T: temperature; t: time of study.
Chemical stability and physical compatibility of meropenem were evaluated at different concentrations and temperatures (Table 1). Admixtures in infusors were stored at 32°C, since it is the physiological temperature reached by the infusor during its administration,16 and at 4.5°C because previous studies indicate that meropenem is more stable under refrigeration than at high temperatures.14 Admixtures in infusion bags were also stored at 4.5°C and at 24.5°C, as room temperature during administration. The possibility of anticipated preparation was evaluated by storing admixtures at 4.5°C followed by 32°C for infusors or 24.5°C for infusion bags; time of refrigeration was greater in admixtures in infusors (12h) than in infusion bags (6h) since its preparation is more complex, takes more time and due to the high workload of nursing staff, it can be prepared during a 12-h shift. Time of study was 5–6 days for infusors and 1 day for infusion bags; the fact of evaluating chemical stability and physical compatibility for more than 1 day in infusors is that its cost is highest and the preparation is most complex so, in case of not administering the infuser to the patient, it can be reused.
Chemical stability of meropenem in each admixture was determined by measuring its concentration by high performance liquid chromatography (HPLC) in an aliquot of 1mL extracted at different sampling times: just after preparation (t=0h) and at 2, 4, 8, 12 and 24h and then every 24h for 5–6 days for admixtures stored in infusors and at 2, 4, 6, 9, 12, 14 and 24h for admixtures in infusion bags.
An Agilent Technologies 1100 HPLC system (Agilent Technologies Inc., Waldbronn, Karlsruhe, Germany) with a diode array detector was used for the analysis. The HPLC method was modified from Foy et al.17 Chromatographic separations were achieved on a reversed phase column C18® (150mm×4.6mm inner diameter; 5μm) with a gradient of mobile phase containing 0.01M NaH2PO4 pH 2 and acetonitrile (t=0min, 93:7, v/v; t=6min, 88:12, v/v; t=16min, 51:49, v/v, respectively) at a flow rate of 0.8mL/min. Column temperature was 25°C and injection volume, 5μL; wavelength was 298nm and time of analysis, 17min. Chromatographic method was previously validated according to the International Conference on Harmonization (ICH) guidelines18 and was adequate to determine meropenem in admixtures.
The remaining concentration of meropenem (%RC) at each sampling time was expressed as the mean percentage and standard deviation (SD) of the initial concentration (after preparation); the initial concentration was expressed as 100%. The stability of a solution for infusion is maintained when the %RC remains >90% throughout the infusion period.19,20 So, the parameter used to define chemical stability was T90, time at which %RC was 90%.
The pair data concentration-time were adjusted, if possible, to a zero- (Eq. (1)) or first-order kinetic equation (Eq. (2)). T90 was estimated by considering the time at which the 95% one-sided confidence limit for the mean curve intersects 90% of the initial concentration of meropenem:
being C, meropenem concentration at a specific sampling time; C0, meropenem concentration at t=0; K0, zero-order degradation rate constant and K1, first-order degradation rate constant; t, sampling time.Physical compatibility of each admixture was evaluated at each sampling time by measuring, in an extracted aliquot of 5mL: (i) pH, with glass electrode and pH-meter (model 3510, Jenway, UK); incompatibility if variation of pH is >5%; (ii) color changes, cloudiness (turbidity) and/or precipitation, measured by visual inspection; incompatibility if some of these parameters appear.
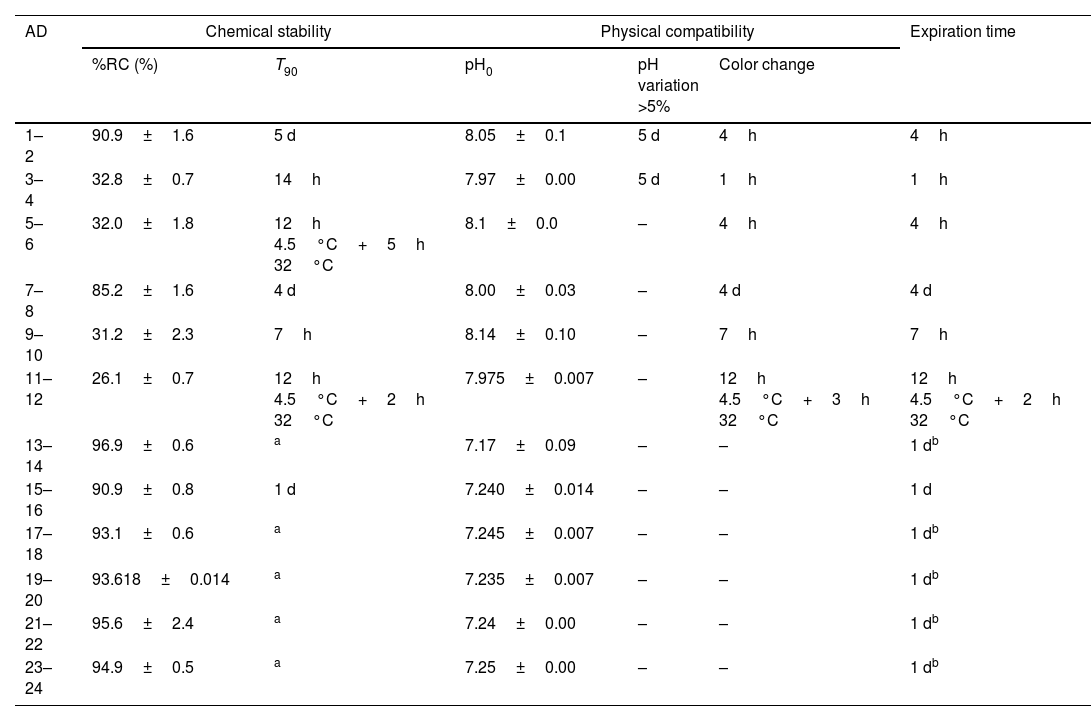
ResultsTable 2 shows chemical stability and physical compatibility parameters for all the admixtures: (a) the average of %RC at the end of the study and the parameter T90; (b) the initial value of pH, time at which variation of pH was >5% and time at which the color of admixture changed; (c) expiration time, by considering chemical stability and physical compatibility.
Chemical stability and physical compatibility parameters of meropenem in admixtures at the end of the study.
| AD | Chemical stability | Physical compatibility | Expiration time | |||
|---|---|---|---|---|---|---|
| %RC (%) | T90 | pH0 | pH variation >5% | Color change | ||
| 1–2 | 90.9±1.6 | 5 d | 8.05±0.1 | 5 d | 4h | 4h |
| 3–4 | 32.8±0.7 | 14h | 7.97±0.00 | 5 d | 1h | 1h |
| 5–6 | 32.0±1.8 | 12h 4.5°C+5h 32°C | 8.1±0.0 | – | 4h | 4h |
| 7–8 | 85.2±1.6 | 4 d | 8.00±0.03 | – | 4 d | 4 d |
| 9–10 | 31.2±2.3 | 7h | 8.14±0.10 | – | 7h | 7h |
| 11–12 | 26.1±0.7 | 12h 4.5°C+2h 32°C | 7.975±0.007 | – | 12h 4.5°C+3h 32°C | 12h 4.5°C+2h 32°C |
| 13–14 | 96.9±0.6 | a | 7.17±0.09 | – | – | 1 db |
| 15–16 | 90.9±0.8 | 1 d | 7.240±0.014 | – | – | 1 d |
| 17–18 | 93.1±0.6 | a | 7.245±0.007 | – | – | 1 db |
| 19–20 | 93.618±0.014 | a | 7.235±0.007 | – | – | 1 db |
| 21–22 | 95.6±2.4 | a | 7.24±0.00 | – | – | 1 db |
| 23–24 | 94.9±0.5 | a | 7.25±0.00 | – | – | 1 db |
AD: admixture; %RC: percentage of remaining concentration of meropenem±standard deviation (%); T90: time at witch %RC is 90%; d: day; h: hour; pH0, initial pH; pH variation, >5%: time at with variation of pH was >5%; color change: time at with color of admixture changed; –: without changes.
As regards admixtures in infusors (admixtures 1–12), the increase in temperature from 4.5°C to 32°C lead to a 64% decrease in %RC at the end of the study and significant decreases in T90 from 5 days to 14h in Singleday® 2mL/h 50mL (120mg/mL) and from 4 days to 7h in Folfusor® LV 10mL/h 240mL (25mg/mL). Similar %RC and T90 values were obtained for admixtures stored at 32°C, with and without pre-cooling. In admixtures with a concentration of meropenem of 120mg/mL (admixtures 1–4), there were variations of pH>5% from day 5at 4.5 and 32°C and visual color changes were observed earliest that in admixtures with low meropenem concentration (admixtures 7–12). So, expiration time was shorter when increasing the concentration of meropenem.
In admixtures stored in Viaflo® (admixtures 13–24), %RC was ≥90% at the end of the study (1 day). Variations of pH were <5% in all cases and visual color changes were not observed. So, expiration time was at least 1 day, since it is the time of the study.
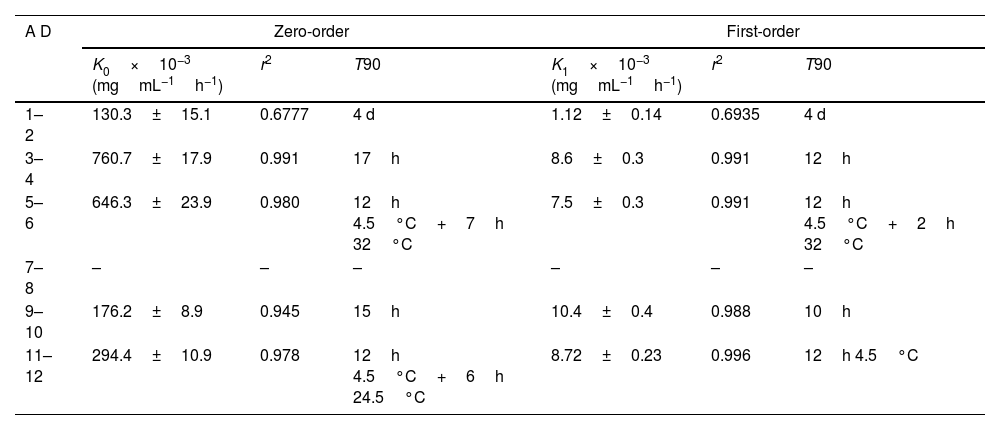
Table 3 shows the kinetic parameters for meropenem degradation and estimated T90 values.
Kinetic parameters for meropenem degradation.
| A D | Zero-order | First-order | ||||
|---|---|---|---|---|---|---|
| K0×10−3 (mgmL−1h−1) | r2 | T90 | K1×10−3 (mgmL−1h−1) | r2 | T90 | |
| 1–2 | 130.3±15.1 | 0.6777 | 4 d | 1.12±0.14 | 0.6935 | 4 d |
| 3–4 | 760.7±17.9 | 0.991 | 17h | 8.6±0.3 | 0.991 | 12h |
| 5–6 | 646.3±23.9 | 0.980 | 12h 4.5°C+7h 32°C | 7.5±0.3 | 0.991 | 12h 4.5°C+2h 32°C |
| 7–8 | – | – | – | – | – | – |
| 9–10 | 176.2±8.9 | 0.945 | 15h | 10.4±0.4 | 0.988 | 10h |
| 11–12 | 294.4±10.9 | 0.978 | 12h 4.5°C+6h 24.5°C | 8.72±0.23 | 0.996 | 12h 4.5°C |
AD: admixture; K0: zero-order degradation rate constant; K1: first-order degradation rate constant; r2: regression coefficient, T90: time at witch %RC is 90%; –: data could not be adjusted to kinetic equations.
Previous studies indicate that the stability of meropenem depends on the diluent, concentration, temperature and pH.17,21 NS is usually used as diluent since it improves stability of meropenem compared to other diluents such as glucose 5% or 10%.13 Stability of meropenem in NS solution decreases with increasing concentration of meropenem17,21 and with increasing temperatures,14,17,21 being stable for a longer time under refrigeration and degrading more quickly at temperatures upper than 35°C.17,21,22 In our study, there were not statistically significant differences in %RC and T90 between admixtures in infusors with different meropenem concentration (admixtures 1–6, 120mg/mL; admixtures 7–12, 25mg/mL) but physical compatibility and consequently, expiration time were reduced at high meropenem concentration and at high temperature (32°C). The observed color changes could be a consequence of the degradation of meropenem by hydrolysis of the beta-lactam ring,19 favored by high concentration and temperature; when color changes, it is recommended not to administer admixtures since physical incompatibility is usually related to loss of potency and increase in toxicity.
So, the only admixture that can be administered in CI of 24h is meropenem 6g in 240mL of NS in Folfusor® LV 10mL/h 240mL as long as it is infused at 4.5°C, being expiration time of 4 days. This admixture becomes a possible alternative in Home Care Units, where EI (administration every 8h and drug withdrawal after 3–4h) is not a viable option. Several studies have proposed different ways to maintain infusors under refrigeration during its administration such as placing the infusors with frozen ice-bricks in a standard carry-bag17,21 or in a cassette attached to an infusion pump22; Grant et al. indicate that it is possible to maintain elastomeric infusion devices at 2–8°C over 24h in a cassette between two ice-bricks, which are changed every 8h, at a room temperature of 20–25°C.22
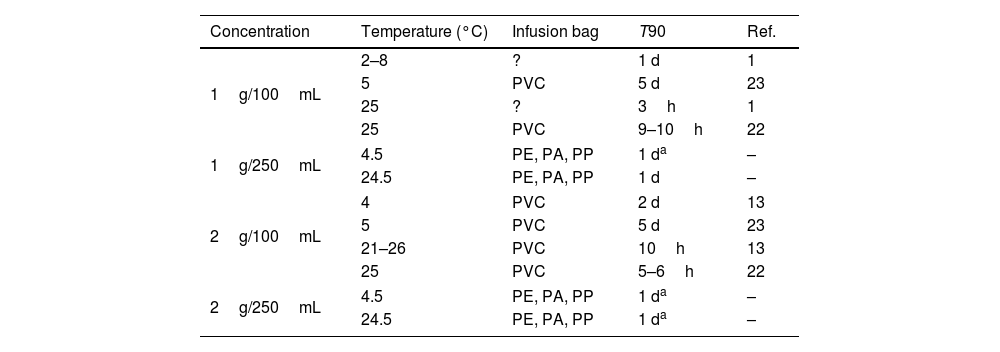
Admixtures containing 1 or 2g of meropenem in 250mL of NS in Viaflo® can be used for II (15–30min) or EI (3–4h) at 4.5 or 24.5°C. Table 4 shows T90 of admixtures of 1 or 2g of meropenem in 100mL and 250mL of NS at room temperature or under refrigeration. As can be observed, increasing the final volume from 100 to 250mL, chemical stability increases up to 1 day at 24.5°C, which would allow EI of meropenem 1 or 2g in 3–4h.
T90 of admixtures of meropenem in infusion bags at different temperatures and concentrations.
| Concentration | Temperature (°C) | Infusion bag | T90 | Ref. |
|---|---|---|---|---|
| 1g/100mL | 2–8 | ? | 1 d | 1 |
| 5 | PVC | 5 d | 23 | |
| 25 | ? | 3h | 1 | |
| 25 | PVC | 9–10h | 22 | |
| 1g/250mL | 4.5 | PE, PA, PP | 1 da | – |
| 24.5 | PE, PA, PP | 1 d | – | |
| 2g/100mL | 4 | PVC | 2 d | 13 |
| 5 | PVC | 5 d | 23 | |
| 21–26 | PVC | 10h | 13 | |
| 25 | PVC | 5–6h | 22 | |
| 2g/250mL | 4.5 | PE, PA, PP | 1 da | – |
| 24.5 | PE, PA, PP | 1 da | – | |
PE: polyethylene; PA: polyamide; PP: polypropilen; ?: unknown; T90: time at witch %RC is 90%; d: days; h: hours; Ref.: reference; –: results obtained in the present study.
Consequently, as previous studies have shown in clinical practice,24–26 the fact of administering meropenem by CI or EI would increase the clinical cure rate by 30% compared to conventional administration of beta-lactams in critical or septic patients. These results are also supported by meta-analysis in critically ill patients or with infections caused by high MIC pathogens, which indicate higher clinical cure rate and lower mortality.27–30
Finally, the possibility of preparation in advance of admixtures of meropenem in NS in infusors and infusion bags was evaluated. The results obtained in this study indicated that preparation in advance is not possible in infusors for CI since after 12h stored at 4.5°C, T90 was 5h at 32°C in Singleday® 2mL/h 50mL and 2h in Folfusor® LV 10mL/h 240mL, being the time of administration to patients of 24h. Nevertheless, preparation in advance is viable in Viaflo® if storing the admixture 6h at 4.5°C and administering during the following 18h at 24.5°C, enough time not only for II (15–30min) but also for EI (3–4h).
In conclusion, the administration of meropenem 6g in perfusion of 24h in 240mL of 0.9% NaCl in infusor Folfusor® LV 10mL/h 240mL could be possible if the admixture is infused at 4.5°C. This study also increases chemical stability and physical compatibility of meropenem 1 or 2g in 0.9% NaCl in Viaflo® infusion bag (250mL) to 24h, being between 3 and 10h1,14 in 100mL; therefore, extended infusion in 3–4h is also feasible, allowing its use in septic or critically patients or with high MIC Gram negative bacteria in which a higher clinical cure rate has been evidenced. Moreover, anticipated preparation of admixtures of meropenem in Viaflo® is possible, as opposed to its usual method of preparation at the time of administration because of its short stability.
As a limitation on this study, the results of chemical stability and physical compatibility of meropenem obtained are applicable to infusion bags made of polyethylene, polyamide and polypropilen (Viaflo®) and infusors of polyisoprene (Singleday® and Folfusor®).
Conflict of interestAuthors state no conflict of interest.