We describe a series of pediatric cases of bacteremia, all of them with a history of heart disease, use of central venous catheter and coinfections. A review of the published literature was carried out in order to enrich the available information.
Material and methodsPediatric observational retrospective study in which three cases of catheter-related bloodstream infection due to Chryseobacterium indologenes were reported in a period of two years in a tertiary care hospital. The analysis was performed with the cases previously reported in the literature.
ResultsThree cases were reported in our center in a period of two years. We found 26 cases reported in the literature. Overall mortality was 26.92% (7/26).
ConclusionsThis microorganism with characteristics of multidrug resistance is associated with the use of medical devices in hospitalized patients. Early identification of this pathogen is crucial to starting treatment.
Describimos una serie de casos pediátricos de bacteriemias, todos ellos con antecedentes de cardiopatías, uso de catéter venoso central y coinfecciones. Se realizó la revisión de la literatura publicada para enriquecer la información disponible.
Material y métodosEstudio retrospectivo observacional pediátrico, en el cual se reportaron 3 casos de bacteriemias asociadas a catéter por Chryseobacterium indologenes en un periodo de 2 años en un hospital de tercer nivel. Se realizó el análisis con los casos previamente reportados en la literatura.
ResultadosSe reportaron 3 casos en nuestro centro en un periodo de 2 años. Se encontraron 26 casos reportados en la literatura. La mortalidad global fue del 26,92% (7/26).
ConclusionesEste microorganismo con características de multirresistencia se asocia al uso de dispositivos médicos en pacientes hospitalizados.
Chryseobacterium indologenes is a Gram-negative, aerobic, non-fermenting multidrug-resistant nosocomial rod that can be found in natural environments such as water and soil.1 Although very rare, when reported, it causes a wide range of infections in hospitalized patients with preexisting conditions or with indwelling devices. The most common presentations are bacteremia, ventilator-associated pneumonia (VAP) and catheter-related bloodstream infection (CRBSI).2
Chryseobacterium spp. infection in children under five years of age is rare.3 According to the literature, only 23 pediatric cases of children with bloodstream infections caused by C. indologenes and associated comorbidities have been reported. Most of them were reported in children with congenital heart diseases or solid tumors.1 The aim of this study was to describe the experience in our center with bloodstream infections by this pathogen in three children with comorbidities and assess literature research. This could offer a better understanding of the treatment of these infections in the pediatric population.
Material and methodsThis observational, descriptive, and retrospective study was conducted in a tertiary care hospital. Data were collected from medical records over a two-year period (2019–2021). Case-patient was defined as a pediatric patient with diagnosis of CRBSI by differential time to positivity (DTP) and associated comorbidities. Adults, previously healthy patients, and infections other than bacteremia were excluded.
Microbiological samples were obtained with the VersaTREK system from peripheral vein and catheter tip. Identification was made using matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS). Susceptibility tests were performed using MicroScan® system (Beckman Coulter, Brea, California, USA) and Clinical and Laboratory Standards Institute (CLSI) 2021 breakpoints for “Non-Enterobacterales”, as there are no breakpoints for this specific pathogen.
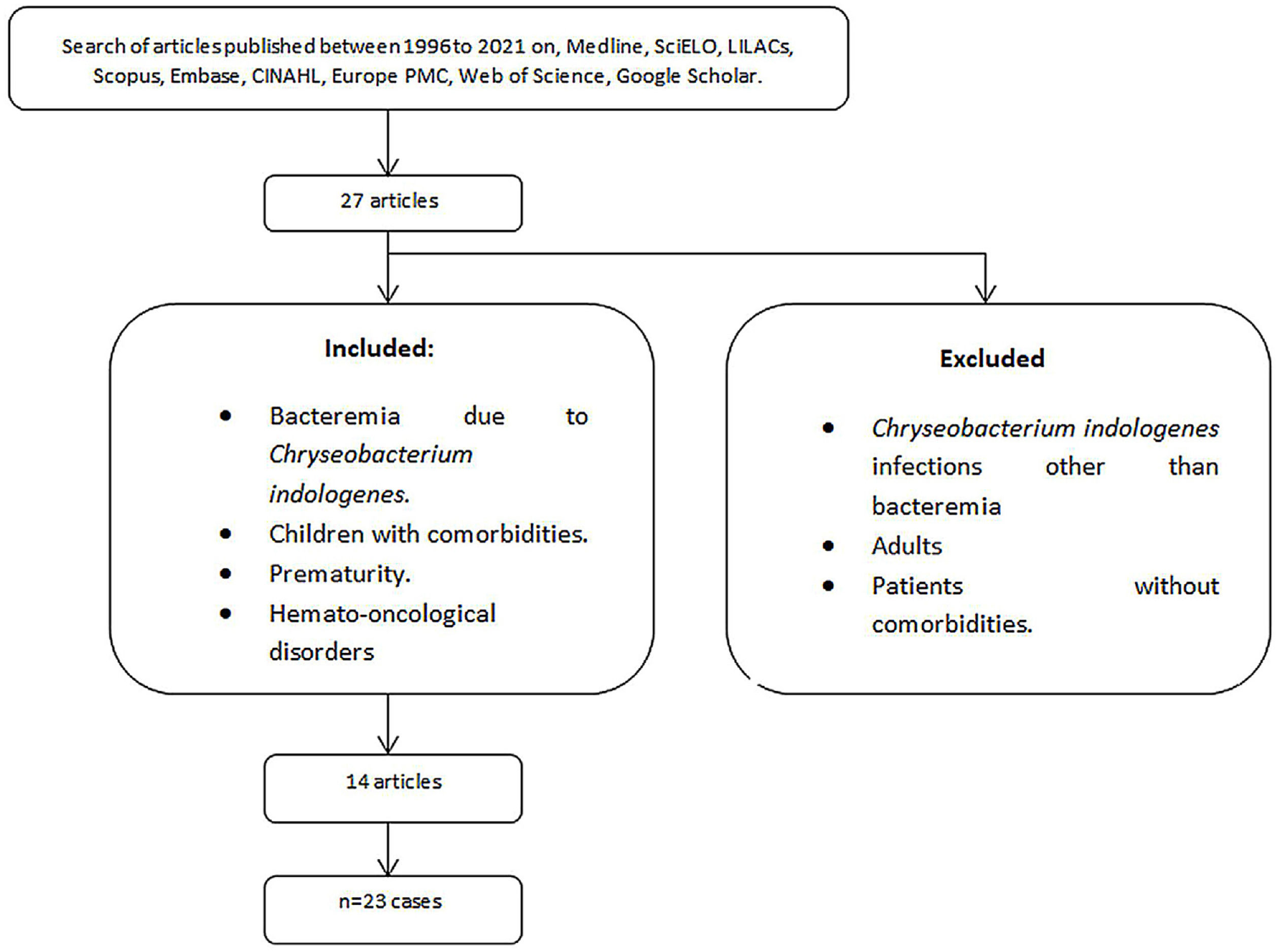
We performed a literature search of core databases including Medline (US National Library of Medicine [NLM]), SciELO (Scientific Electronic Library Online), LILACS (Latin American and Caribbean Literature in Health Science), Scopus (Elsevier, Amsterdam, Netherland), the Excerpta Medica Database (Embase [Elsevier, Amsterdam, Netherlands]), Cumulative Index to Nursing and Allied Health Literature (CINAHL [EBSCO, Ipswich, Massachusetts]), Europe PMC (European Bioinformatics Institute) and Web of Science (Clarivate Analytics, Philadelphia, Pennsylvania) between 1985 and 2021 using the keywords: “Chryseobacterium spp.”, “Chryseobacterium species” “C.indologenes” “comorbidities, “pediatric patient”, “children”, “cancer”, “bacteremia” “bloodstream infection” to identify case reports and case series. References of publications were reviewed to recognize duplicate reports.
ResultsCase 1One-year-old male with medical history of anomalous pulmonary venous connection, post operated by Baffle surgery and pericardial patching was admitted for exacerbation of pulmonary hypertension associated with stenosis of the left mitral anastomosis and Ross grade II congestive heart failure.
After 20 days of admission, the patient developed pneumonia and was transferred to the pediatric intensive care unit (PICU). Empirical treatment was started and after ten days, he was discharged to the pediatric ward due to clinical improvement. Three days later he had septic shock signs: fever, chills, tachycardia, leukocytosis, neutrophilia, and C-Reactive Protein (CRP) 11.6mg/dl; meropenem intravenous (IV) (60mg/kg/day) scheme was started.
The following day he suffered cardiorespiratory arrest and advanced resuscitation maneuvers were performed with no success. Two days later, a post-mortem diagnosis of CRBSI was made in which Serratia spp. and C. indologenes were isolated. C. indologenes showed susceptibility to cefepime, ciprofloxacin, imipenem, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX), and resistance to ceftazidime and gentamicin.
Case 2Newborn female with Turner syndrome with coarctation of the aorta, aortic stenosis, tricuspid insufficiency, and persistence of the left vena cava was hospitalized since birth for prostaglandins management and surgical programming.
She underwent coarctectomy and aortoplasty surgery; in the immediate postoperative period she suffered hemorrhagic shock, bilateral chylothorax, acute kidney injury and VAP, which led to empirical management with meropenem plus vancomycin IV; blood cultures were ordered.
C. indologenes grew in peripheral blood cultures and was susceptible to fluoroquinolones, TMP-SMX, piperacillin/tazobactam, and resistant to cephalosporins, gentamicin and meropenem, requiring the administration of ciprofloxacin IV for seven days.
One day later, clinical worsening appeared: urine culture revealed an extended-spectrum betalactamase (ESBL)-producing Klebsiella pneumoniae with >100,000 colony forming units (CFU)/ml, and CRBSI diagnosis made by DTP isolated Stenotrophomonas maltophilia and C. indologenes. The latter was susceptible to fluoroquinolones, piperacillin/tazobactam, and resistant to cephalosporins, carbapenems, and aminoglycosides. S. maltophilia treatment of choice is TMP-SMX, but as our center did not have this drug, and with the purpose of treating the C. indologenes co-infection as well, treatment with levofloxacin IV was initiated.
One day later, on her 109th day of life and hospitalization, she presented sudden cardiac arrest requiring advanced resuscitation maneuvers without spontaneous circulation response, resulting in death due to cardiogenic-septic shock.
Case 3A 6-year-old male with medical history of Down's syndrome and atrial communication, severe malnutrition, chronic renal failure with bilateral hydronephrosis, and scabies was admitted to the hospital for central venous catheter (CVC) placement, parenteral nutrition administration, and monitoring.
On his fifth day of hospitalization, he developed fever and rhinorrhea. Polymerase-chain-reaction (PCR) from nasopharyngeal swab for SARS-CoV-2 was positive, requiring symptomatic management. Three days later Candida albicans CRBSI diagnosis was made and therapy with fluconazole IV (12mg/kg/day) was provided. After 14 days with fluconazole, the patient presented fever, blood cultures were taken in this approach, revealing a Gram-negative bacillus, the CVC was removed and therapy with meropenem IV started. Three days after, C. indologenes was identified by DTP. Susceptibility to TMP-SMX and levofloxacin was demonstrated, as well as resistance to amikacin, cephalosporins, and carbapenems, and antibiotic therapy was changed to levofloxacin IV (10mg/kg/12h) and seven days of therapy were completed without complications. He was discharged after 29 days of hospitalization.
A total of 27 articles reported C. indologenes infections. Gathering a total of 39 cases, 13 were excluded because they were infections other than bacteremia or in previously healthy patients; from the remaining studies, three cases were excluded because patients were adults. A total of 23 C. indologenes bacteremia cases were identified and analyzed together with ours (Fig. 1). Overall mortality was 26.92% (7/26).
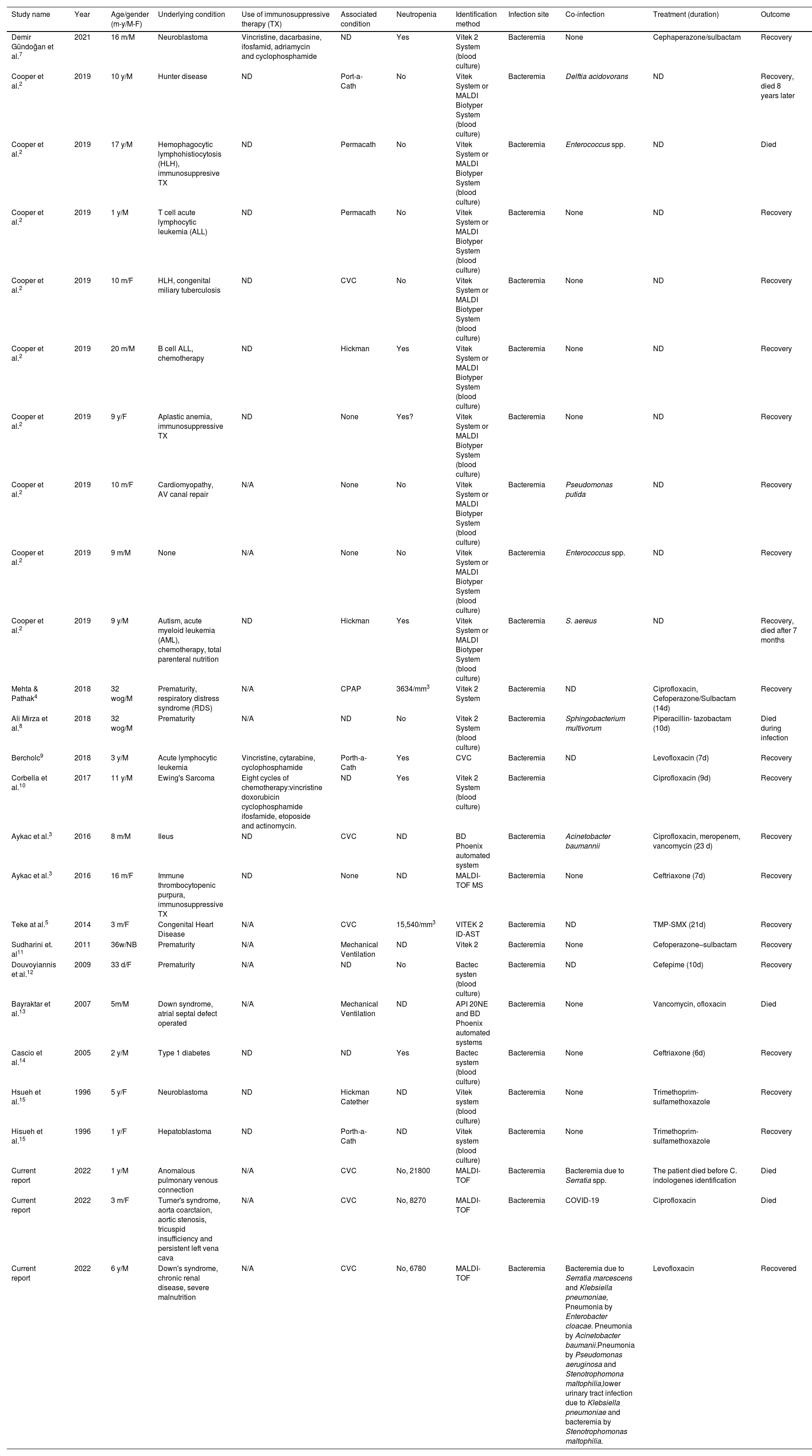
Coinfections, underlying and associated conditions, diagnostic methods, and treatments are described in Table 1.
Summary of Chryseobacterium indologenes bacteremias in children, coinfection, underlying conditions, diagnostic methods, and treatment.
| Study name | Year | Age/gender (m-y/M-F) | Underlying condition | Use of immunosuppressive therapy (TX) | Associated condition | Neutropenia | Identification method | Infection site | Co-infection | Treatment (duration) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demir Gündoğan et al.7 | 2021 | 16 m/M | Neuroblastoma | Vincristine, dacarbasine, ifosfamid, adriamycin and cyclophosphamide | ND | Yes | Vitek 2 System (blood culture) | Bacteremia | None | Cephaperazone/sulbactam | Recovery |
| Cooper et al.2 | 2019 | 10 y/M | Hunter disease | ND | Port-a-Cath | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | Delftia acidovorans | ND | Recovery, died 8 years later |
| Cooper et al.2 | 2019 | 17 y/M | Hemophagocytic lymphohistiocytosis (HLH), immunosuppresive TX | ND | Permacath | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | Enterococcus spp. | ND | Died |
| Cooper et al.2 | 2019 | 1 y/M | T cell acute lymphocytic leukemia (ALL) | ND | Permacath | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | None | ND | Recovery |
| Cooper et al.2 | 2019 | 10 m/F | HLH, congenital miliary tuberculosis | ND | CVC | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | None | ND | Recovery |
| Cooper et al.2 | 2019 | 20 m/M | B cell ALL, chemotherapy | ND | Hickman | Yes | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | None | ND | Recovery |
| Cooper et al.2 | 2019 | 9 y/F | Aplastic anemia, immunosuppressive TX | ND | None | Yes? | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | None | ND | Recovery |
| Cooper et al.2 | 2019 | 10 m/F | Cardiomyopathy, AV canal repair | N/A | None | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | Pseudomonas putida | ND | Recovery |
| Cooper et al.2 | 2019 | 9 m/M | None | N/A | None | No | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | Enterococcus spp. | ND | Recovery |
| Cooper et al.2 | 2019 | 9 y/M | Autism, acute myeloid leukemia (AML), chemotherapy, total parenteral nutrition | ND | Hickman | Yes | Vitek System or MALDI Biotyper System (blood culture) | Bacteremia | S. aereus | ND | Recovery, died after 7 months |
| Mehta & Pathak4 | 2018 | 32 wog/M | Prematurity, respiratory distress syndrome (RDS) | N/A | CPAP | 3634/mm3 | Vitek 2 System | Bacteremia | ND | Ciprofloxacin, Cefoperazone/Sulbactam (14d) | Recovery |
| Ali Mirza et al.8 | 2018 | 32 wog/M | Prematurity | N/A | ND | No | Vitek 2 System (blood culture) | Bacteremia | Sphingobacterium multivorum | Piperacillin- tazobactam (10d) | Died during infection |
| Bercholc9 | 2018 | 3 y/M | Acute lymphocytic leukemia | Vincristine, cytarabine, cyclophosphamide | Porth-a-Cath | Yes | CVC | Bacteremia | ND | Levofloxacin (7d) | Recovery |
| Corbella et al.10 | 2017 | 11 y/M | Ewing's Sarcoma | Eight cycles of chemotherapy:vincristine doxorubicin cyclophosphamide ifosfamide, etoposide and actinomycin. | ND | Yes | Vitek 2 System (blood culture) | Bacteremia | Ciprofloxacin (9d) | Recovery | |
| Aykac et al.3 | 2016 | 8 m/M | Ileus | ND | CVC | ND | BD Phoenix automated system | Bacteremia | Acinetobacter baumannii | Ciprofloxacin, meropenem, vancomycin (23 d) | Recovery |
| Aykac et al.3 | 2016 | 16 m/F | Immune thrombocytopenic purpura, immunosuppressive TX | ND | None | ND | MALDI-TOF MS | Bacteremia | None | Ceftriaxone (7d) | Recovery |
| Teke at al.5 | 2014 | 3 m/F | Congenital Heart Disease | N/A | CVC | 15,540/mm3 | VITEK 2 ID-AST | Bacteremia | ND | TMP-SMX (21d) | Recovery |
| Sudharini et. al11 | 2011 | 36w/NB | Prematurity | N/A | Mechanical Ventilation | ND | Vitek 2 | Bacteremia | None | Cefoperazone–sulbactam | Recovery |
| Douvoyiannis et al.12 | 2009 | 33 d/F | Prematurity | N/A | ND | No | Bactec systen (blood culture) | Bacteremia | ND | Cefepime (10d) | Recovery |
| Bayraktar et al.13 | 2007 | 5m/M | Down syndrome, atrial septal defect operated | N/A | Mechanical Ventilation | ND | API 20NE and BD Phoenix automated systems | Bacteremia | None | Vancomycin, ofloxacin | Died |
| Cascio et al.14 | 2005 | 2 y/M | Type 1 diabetes | ND | ND | Yes | Bactec system (blood culture) | Bacteremia | None | Ceftriaxone (6d) | Recovery |
| Hsueh et al.15 | 1996 | 5 y/F | Neuroblastoma | ND | Hickman Catether | ND | Vitek system (blood culture) | Bacteremia | None | Trimethoprim-sulfamethoxazole | Recovery |
| Hisueh et al.15 | 1996 | 1 y/F | Hepatoblastoma | ND | Porth-a-Cath | ND | Vitek system (blood culture) | Bacteremia | None | Trimethoprim-sulfamethoxazole | Recovery |
| Current report | 2022 | 1 y/M | Anomalous pulmonary venous connection | N/A | CVC | No, 21800 | MALDI-TOF | Bacteremia | Bacteremia due to Serratia spp. | The patient died before C. indologenes identification | Died |
| Current report | 2022 | 3 m/F | Turner's syndrome, aorta coarctaion, aortic stenosis, tricuspid insufficiency and persistent left vena cava | N/A | CVC | No, 8270 | MALDI-TOF | Bacteremia | COVID-19 | Ciprofloxacin | Died |
| Current report | 2022 | 6 y/M | Down's syndrome, chronic renal disease, severe malnutrition | N/A | CVC | No, 6780 | MALDI-TOF | Bacteremia | Bacteremia due to Serratia marcescens and Klebsiella pneumoniae, Pneumonia by Enterobacter cloacae. Pneumonia by Acinetobacter baumanii.Pneumonia by Pseudomonas aeruginosa and Stenotrophomona maltophilia,lower urinary tract infection due to Klebsiella pneumoniae and bacteremia by Stenotrophomonas maltophilia. | Levofloxacin | Recovered |
Abbreviations: M: male; F: female; N/A: not applicable, WOG: weeks of gestation, m: months, y: years, PCR: polymerase chain reaction; MALDI-TOF MS: matrix-assisted laser desorption/ionization time-of-flight mass spectrophotometry, ND: no data, CVC: central venous catheter, ALL: acute lymphocytic leukemia, HLH: hemophagocytic lymphohistiocytosis, AML: acute myeloid leukemia, RDS: respiratory distress syndrome, TX: therapy.
The genus Chryseobacterium, belonging to the Flavobacteriaceae family, was described in 1994, and species that are most frequently isolated are C. multivorum, C. indologenes, C. odoratum, C. gleum, and C. breve.3 These are Gram-negative rod-shaped bacilli that form yellow-pigmented colonies in culture due to the production of the pigment flexirubin and that are normally found in water and soil.4,5C. indologenes is commonly isolated from medical devices that are in contact with water as intubation tubes, respirators, peritoneal and central venous catheters, and chest drains; they can cause invasive infections in humans.4,5 In 2018, Cantero et al. reported an outbreak of this pathogen in 12 critically ill patients, a hypothesis was that the cause of the outbreak was the contamination of handwashing sinks and drains.6 In our center, the cases were not related in time, therefore it cannot be considered an outbreak.
Immunocompromised hosts and newborns are the main groups these opportunistic pathogens infect.1 Nosocomial pneumonia, bacteremia, CRBSI, biliary tract infection, meningitis, and wound infection are among the clinical manifestations that have been reported.4
C. indologenes multidrug-resistant (MDR) characteristic is due to the production of beta-lactamases (IND-3)4 capable of hydrolyzing all beta-lactams, except for monobactams. This makes it difficult to choose the optimal antibiotic treatment, and there are currently no guidelines. Besides TMP-SMZ, quinolones are also active against C. indologenes.4
The epidemiology of our center reports an ESBL prevalence greater than 15%, the reason why the empirical treatment for healthcare-associated sepsis is carbapenems, which were administered in our three cases. After the C. indologenes isolation, we conducted the literature research, and as described above, this pathogen has an MDR characteristic, and has been demonstrated that quinolones are also active against it.4 Therefore, we decided to use IV quinolones to treat the two patients in whom C. indologenes was isolated in a timely manner.
There is no consensus on how to effectively treat C. indologenes and its multiresistant characteristic creates a complex approach to both empirical and specific management.
Empirical management of bacteremia should be according to the local epidemiology of each center and the medical history of the patient; our cases were susceptible to quinolones; the only patient who survived was treated with levofloxacin. The identification of the causal agent in a timely manner is vital for the correct management of patients. More research about the clinical and pharmaceutical management of C. indologenes in pediatric patients is still needed.
Consent for publicationWritten informed consent for publication of clinical details was obtained from the parents of the patient. A copy of the consent form is available for review by the editor of the journal.
Financial support and disclosureThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.