Haemophilus influenzae is responsible of community-acquired respiratory tract and otolaryngology infections. First line treatment is based on betalactam antibiotics (aminopenicillins and cephalosporins), although fluoroquinolones (FQ) are widely used among adults. Resistance to these compounds remains relatively rare among H. influenzae strains. Chromosomal point mutations in the quinolone resistance-determining regions of the genes encoding DNA gyrase (gyr A and gyr B) and topoisomerase IV (parC and parE) constitute the main FQ resistance mechanisms in H. influenzae.1 Other mechanisms such as efflux pumps and porin loss have been suggested to be involved in the resistance of these compounds.2
Between January 2014 and March 2017, a total of 895 clinical strains of H. influenzae were isolated from various clinical specimens at the Microbiology Laboratory of the University Hospital of Álava (Vitoria-Gasteiz, Spain). Ciprofloxacin (CIP) susceptibility data for all these strains was recorded and is currently being analysed in order to determine the CIP-resistant rates among H. influenzae and its relationship with the patients age. For this evaluation, one isolate per patient was considered.
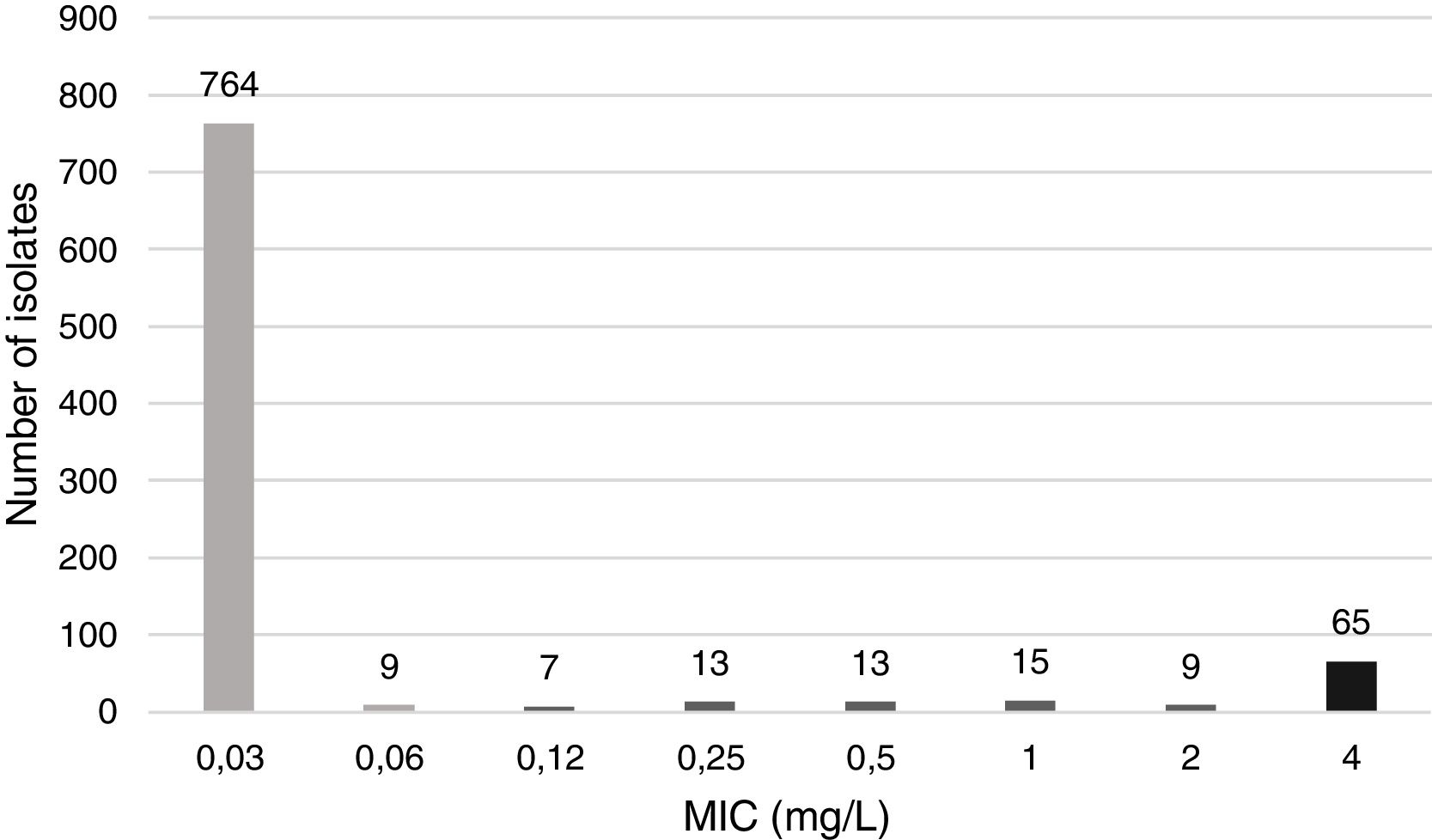
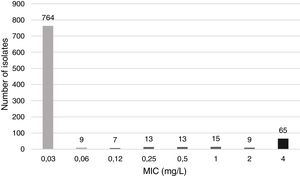
Minimum inhibitory concentration (MIC) of CIP was determined by the microdilution method with Haemophilus test medium (HTM) and commercial panels (STRHAE2, Sensititre, West Sussex, England). H. influenzae ATCC 49247 was used as a susceptible control strain. MIC distribution of CIP is displayed in Fig. 1.
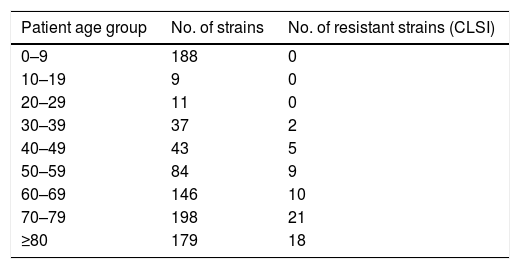
Following Clinical and Laboratory Standards Institute (CLSI) interpretative criteria,3 which defines the CIP susceptibility breakpoint at ≤1mg/L, 65 (7.3%) of the strains evaluated were CIP non-susceptible. All CIP-resistant strains following CLSI were isolated from patients that were over 36 years old (mean age of 70.1); none were isolated in children (Table 1). This could be explained by the avoidance of FQ use among paediatric population due to toxicity concerns.
Major differences between the European Committee on Antimicrobial Susceptibility Testing4 (EUCAST) and the CLSI in terms of culture media (Mueller-Hinton agar+5% defibrinated horse blood and 20mg/L β-NAD (MH-F), compared to HTM) and breakpoints for susceptibility testing of H. influenzae make it almost impossible to come to a harmonized conclusion when comparing studies following recommendations by either committee.
Thus, if EUCAST criteria in terms of CIP susceptibility breakpoint (≤0.06mg/L) and irrespective of the medium were applied, 122 (13.6%) strains would be considered resistant. These results suggest that the adoption of CLSI CIP susceptibility breakpoint of ≤1mg/L might lead to under recognition of low-level CIP-resistance H. influenzae strains.
In our analysis, a high percentage of FQ-resistant H. influenzae were found compared to previous studies.5 This could be due to the extended use of these compounds in Spain. Our results are intended to rise the concern about the increase of FQ-resistance among H. influenzae and to highlight the importance of surveillance programmes to control de emergence of this type of strains in order to avoid therapeutic failures. Harmonized criteria for susceptibility testing and interpretation are needed to provide accurate advice to clinicians as well as to obtain reliable epidemiological information at local, regional or national levels.