Sexually transmitted diseases (STDs) are one of the most common and universal problems of public health. Human immunodeficiency virus (HIV) and STD infections are clearly interrelated, and share risks, incidence and transmission mechanisms.
The aim of this document is to inform health professionals about the current situation and management of STDs, which, because of their relevance, need greater care, both in the general population and in the population with HIV.
These guidelines for STD treatment, although clinically oriented and focused especially on treatment, contain other aspects related to assessing and evaluating patients, as well as recommendations for diagnosis.
Las infecciones de transmisión sexual (ITS) son uno de los problemas más frecuentes y universales de Salud Pública. La infección por el virus de la inmunodeficiencia humana y las ITS están claramente interrelacionadas, compartiendo riesgos, incidencia y mecanismos de transmisión.
El objetivo de este documento es dar a conocer a los profesionales sanitarios la situación actual y el manejo de aquellas ITS, que por su relevancia necesitan una mayor atención, tanto en población general como en población infectada por el virus de la inmunodeficiencia humana.
Estas directrices para el tratamiento de las ITS, aunque orientadas desde el punto de vista clínico y centrado especialmente en el tratamiento, también recogen otros aspectos relacionados con la evaluación y valoración del paciente así como recomendaciones de diagnóstico.
Sexually transmitted diseases (STD) are one of the most frequent and universal problems of Public Health. Their high morbidity and the possibility of medium and long term sequelae demands health professionals to have basic but sufficient knowledge to manage them properly, both for their prevention and approach as for their diagnosis, treatment, contact search and monitoring. Infection by human immunodeficiency virus (HIV) and STD are clearly interrelated, sharing risks, incidence and transmission mechanisms.
These guidelines for STD treatment, although oriented from a clinical viewpoint and focused especially on treatment, gather other aspects related to assessment and evaluation of the patient, as well as recommendations for diagnosis.1
Recommendations contained in this guide are based on scientific evidence and on expert opinion. Each recommendation is rated with a letter indicating its power [A (should always be offered), B (should generally be offered) or C (should optionally be offered) and a number expressing the evidence supporting said recommendation [I (results obtained from one or more randomized clinical trials on clinical or laboratory aspects or from a meta-analysis); II (from one or more non-randomized clinical trials or observational data from cohorts); and III (expert opinion)].
General measures for STD prevention and controlThe assessment of STD individual risk requires compiling a medical history including questions about sexual behavior and other risk factors.
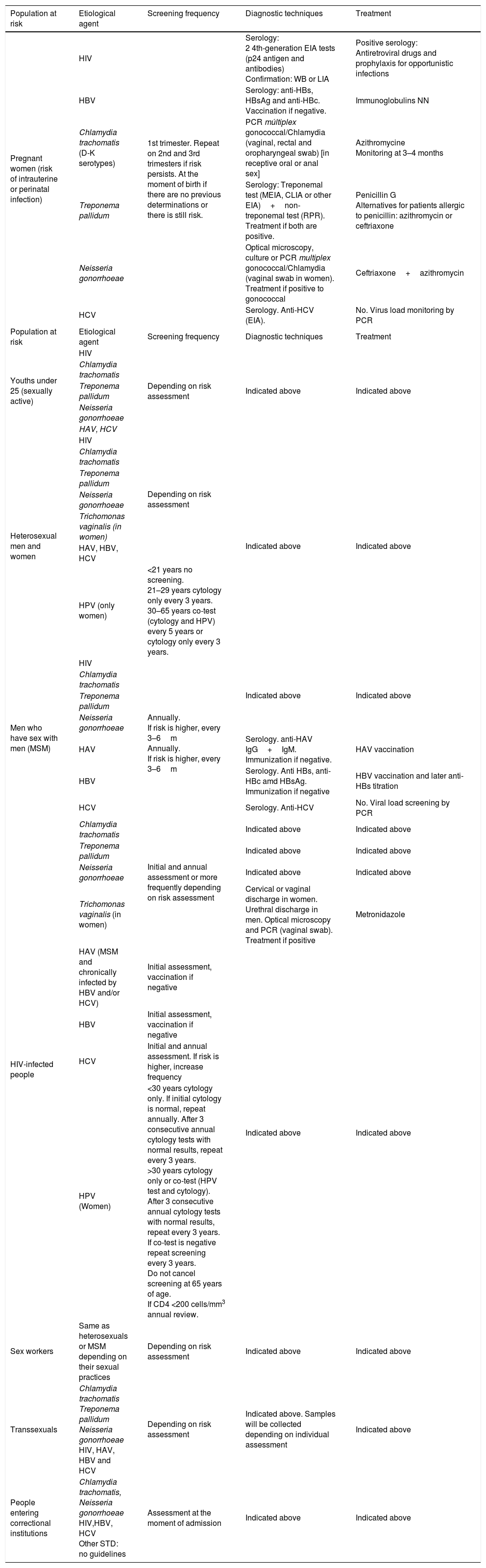
Table 1 shows the most common STD screenings based on populations at risk. An HIV infection should be ruled out in all populations with STD risk practices.
Most-frequent STD screening by populations at risk.
| Population at risk | Etiological agent | Screening frequency | Diagnostic techniques | Treatment |
|---|---|---|---|---|
| Pregnant women (risk of intrauterine or perinatal infection) | HIV | 1st trimester. Repeat on 2nd and 3rd trimesters if risk persists. At the moment of birth if there are no previous determinations or there is still risk. | Serology: 2 4th-generation EIA tests (p24 antigen and antibodies) Confirmation: WB or LIA | Positive serology: Antiretroviral drugs and prophylaxis for opportunistic infections |
| HBV | Serology: anti-HBs, HBsAg and anti-HBc. Vaccination if negative. | Immunoglobulins NN | ||
| Chlamydia trachomatis (D-K serotypes) | PCR múltiplex gonococcal/Chlamydia (vaginal, rectal and oropharyngeal swab) [in receptive oral or anal sex] | Azithromycine Monitoring at 3–4 months | ||
| Treponema pallidum | Serology: Treponemal test (MEIA, CLIA or other EIA)+non-treponemal test (RPR). Treatment if both are positive. | Penicillin G Alternatives for patients allergic to penicillin: azithromycin or ceftriaxone | ||
| Neisseria gonorrhoeae | Optical microscopy, culture or PCR multiplex gonococcal/Chlamydia (vaginal swab in women). Treatment if positive to gonococcal | Ceftriaxone+azithromycin | ||
| HCV | Serology. Anti-HCV (EIA). | No. Virus load monitoring by PCR | ||
| Population at risk | Etiological agent | Screening frequency | Diagnostic techniques | Treatment |
| Youths under 25 (sexually active) | HIV | Depending on risk assessment | Indicated above | Indicated above |
| Chlamydia trachomatis | ||||
| Treponema pallidum | ||||
| Neisseria gonorrhoeae | ||||
| HAV, HCV | ||||
| Heterosexual men and women | HIV | Depending on risk assessment | Indicated above | Indicated above |
| Chlamydia trachomatis | ||||
| Treponema pallidum | ||||
| Neisseria gonorrhoeae | ||||
| Trichomonas vaginalis (in women) | ||||
| HAV, HBV, HCV | ||||
| HPV (only women) | <21 years no screening. 21–29 years cytology only every 3 years. 30–65 years co-test (cytology and HPV) every 5 years or cytology only every 3 years. | |||
| Men who have sex with men (MSM) | HIV | Annually. If risk is higher, every 3–6m Annually. If risk is higher, every 3–6m | Indicated above | Indicated above |
| Chlamydia trachomatis | ||||
| Treponema pallidum | ||||
| Neisseria gonorrhoeae | ||||
| HAV | Serology. anti-HAV IgG+IgM. Immunization if negative. | HAV vaccination | ||
| HBV | Serology. Anti HBs, anti-HBc amd HBsAg. Immunization if negative | HBV vaccination and later anti-HBs titration | ||
| HCV | Serology. Anti-HCV | No. Viral load screening by PCR | ||
| HIV-infected people | Chlamydia trachomatis | Initial and annual assessment or more frequently depending on risk assessment | Indicated above | Indicated above |
| Treponema pallidum | Indicated above | Indicated above | ||
| Neisseria gonorrhoeae | Indicated above | Indicated above | ||
| Trichomonas vaginalis (in women) | Cervical or vaginal discharge in women. Urethral discharge in men. Optical microscopy and PCR (vaginal swab). Treatment if positive | Metronidazole | ||
| HAV (MSM and chronically infected by HBV and/or HCV) | Initial assessment, vaccination if negative | Indicated above | Indicated above | |
| HBV | Initial assessment, vaccination if negative | |||
| HCV | Initial and annual assessment. If risk is higher, increase frequency | |||
| HPV (Women) | <30 years cytology only. If initial cytology is normal, repeat annually. After 3 consecutive annual cytology tests with normal results, repeat every 3 years. >30 years cytology only or co-test (HPV test and cytology). After 3 consecutive annual cytology tests with normal results, repeat every 3 years. If co-test is negative repeat screening every 3 years. Do not cancel screening at 65 years of age. If CD4 <200 cells/mm3 annual review. | |||
| Sex workers | Same as heterosexuals or MSM depending on their sexual practices | Depending on risk assessment | Indicated above | Indicated above |
| Transsexuals | Chlamydia trachomatis Treponema pallidum Neisseria gonorrhoeae HIV, HAV, HBV and HCV | Depending on risk assessment | Indicated above. Samples will be collected depending on individual assessment | Indicated above |
| People entering correctional institutions | Chlamydia trachomatis, Neisseria gonorrhoeae HIV,HBV, HCV Other STD: no guidelines | Assessment at the moment of admission | Indicated above | Indicated above |
- 1.
It is recommended to conduct a screening of STD in sexually active persons who are members of groups with a high STD prevalence, like youths under age 25, men who have sex with men (MSM), sex workers, adolescents and people entering correctional institutions (A-II).
- 2.
It is recommended to conduct a screening of STD in patients with HIV infection at the initial assessment and, later, on an annual basis if they are sexually active or more frequently depending on the individual risk evaluation (A-II).
- 3.
It is recommended to conduct a screening of STD in pregnant women during their first trimester of pregnancy, and if it proves to be negative, repeat it before and at the time of childbirth, depending on the woman's risk situation and practices. It should also be performed at the time of childbirth in pregnant women who have missed their prenatal checks, especially women from high-prevalence countries (B-II).
- 4.
STD screening includes performing serological tests and detecting Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis in all locations possible depending on the kind of sexual practices (A-II). Serological screening will initially include HIV, lues, HBV and HCV serology in every patient with risk factors and HAV serology in MSM or patients with risk practices for this infection.
- 5.
Screening frequency will depend on the individual risk assessment. Patients with negative HAV and HBV serology will have to get vaccinated against both viruses (A-II).
- 6.
It is recommended to perform HPV-infection screening and rule out carcinoma of the uterine cervix in:
- (a)
Women without HIV infection depending on the age:
- (i)
<21 years old, no screening. (A-III).
- (ii)
21–29 years old, only cytology every 3 years. (A-III).
- (iii)
30–65 years old, co-test (cytology and HPV) every 5 years (preferred option) or only cytology every 3 years (acceptable option) (A-II).
- (i)
- (b)
HIV-positive women
- (i)
In HIV-positive women and adolescents, cervical cancer screening with cytology only should begin within the year of onset of sexual activity. If they are already sexually active, within the first year after HIV diagnosis but no later than age 21 (A-II).
- (ii)
<30 years old, only cytology. If the first cytology is normal, repeat it in one year. After 3 yearly consecutive normal cytology tests, repeat them every 3 years (A-II).
- (iii)
>30 years cytology only or co-test (HPV test or cytology). After 3 yearly consecutive normal cytology tests, repeat every 3 years. If co-test is negative (normal cytology and negative HPV), repeat screening every 3 years (A-II).
- (iv)
Do not cancel screening at 65 years of age (A-II).
- (v)
In women with CD4<200cell/mm3, an annual examination with cytology will be performed (A-II).
- (vi)
Women with HIV who have cervical cytological findings with low-grade, or worse, squamous intraepithelial lesions should be referred to get a colposcopy (A-II).
- (i)
- (a)
Safe-sex information should be part of all sexual health consultations.
Recommendation- 1.
Safe-sex information should be part of all sexual health consultations and should include information about ways of transmission and the risks of certain sexual practices, as well as condoms’ effectiveness and limitations (A-III).
There are three basic partner notification procedures: through the patient or “patient referral” when notification is made by the index case; “provider referral” when it is made by a health professional, and “contract referral”, where the patient assumes notification but if after a certain time he/she has not done so, notification is then in the hands of a health professional. There is no clear evidence on which is the most effective procedure for the different STD.
Recommendations- 1.
Contacts study should be performed as early as possible, with the aim of minimizing reinfections and preventing progression of the disease in infected couples. Other STD should be ruled out, in addition to performing the appropriate tests to diagnose the infection the patient has been exposed to, (A-III).
- 2.
The choice of method will depend on the availability of resources and the acceptability of the patient and his/her contacts. If it is possible, “contract referral” is recommended; if it is not, it is recommended to conduct, at least, “patient referral”, strengthened by giving the patient written material (A-III).
STD prevention through vaccination is limited to hepatitis A and B and HPV vaccines. (See Consensus Document on Post-Exposure Occupational and Non-Occupational Prophylaxis in respect to HIV, HBV and HCV, available at (in Spanish):
Recommendations- 1.
It is necessary to know the patient's immunization history to offer vaccination to those who need it according to the current immunization schedule (A-III).
- 2.
In patients with HIV infection, vaccination against HAV and HBV is indicated in all susceptible patients (antiHBs and antiHBc negative) with undetectable HIV viral load and lymphocytes CD4 >200cells/mm3(A-I).
- 3.
HAV vaccination requires 2 doses with a 6-to-8-month interval (A-I). It is encouraged to administer a third dose if there is no antibody production one month after the second dose (B-II).
- 4.
In patients with HIV infection, it is recommended to administer four double doses of HBV vaccine (40μg) on months 0, 1, 2 and 6 (A-I).
It should be characterized by being comprehensive and detailed. Most commonly, the patient's history is reviewed “by systems”, with special attention to skin, mucous membranes and skin appendages. Physical examination must be equally comprehensive.
To test a culture, samples must be collected: before establishing anti-microbe treatment, from active lesions. They must be transported without delay and stored at the appropriate temperature conditions for each case. When using nucleic acids amplification techniques (NAT), the conditions for collecting, transporting and storing are less strict.
Recommendations- 1.
It is recommended to aim the anamnesis at finding acute symptoms. It should cover the different STD semiology, asking for the onset of the symptoms and the time elapsed between the unprotected sexual relations and their onset (A-II).
- 2.
It is recommended that physical examination is comprehensive, because some STD, especially secondary syphilis, may manifest in different anatomic regions (B-III).
- 3.
It is recommended to offer patients the attendance of an interpreter in the case of a language barrier, of another health professional for the intimate examination as well as the possibility to choose the gender of the doctor who will take care of him/her whenever possible (B-III).
- 4.
It is recommended to avoid the use of lubricants when collecting vaginal samples, since they are toxic for gonococcus (A-II).
- 5.
The use of NAT for screening allows women to collect their own vaginal discharge and men to collect their own urine. Therefore, it is the recommended technique when collection is made by the patient itself (A-I).
STD are the most common cause for infectious urethritis and cervicitis. It is frequent to find coinfection by various agents, Neisseria gonorrhoeae and Chlamydia trachomatis are mostly involved. Other microorganisms are Mycoplasma genitalium (with a growing interest) Trichomonas vaginalis, Adenovirus or HSV. Regarding symptoms, urethritis is characterized by urethral inflammation, presenting dysuria, discomfort and mucous, mucopurulent or purulent urethral discharge as the most frequent symptoms, but asymptomatic cases may also be found. Cervicitis, in contrast, will be frequently asymptomatic, its main signs being mucopurulent cervical discharge or cervical bleeding during the collection of samples or during sexual relations.
Diagnosis will be made on the basis of symptoms and compatible physical examination, real-time PCR and/or culture of the different agents in urethral/cervical discharge. Treatment should start at the moment of diagnosis of the pathology if Gram is conclusive, without waiting for microbiological results.
Recommendations- 1.
It is recommended to start syndromic treatment at the initial visit in patients where monitoring cannot be guaranteed (C-III).
- 2.
In the case of gonococcal infection and due to the growing resistance to cephalosporins and flaws detected in the treatment, dual treatment is recommended as the choice against uncomplicated gonococcal infections (B-II). In the case of cervicitis or NGU, doxycycline is the regimen of choice (A-II). A long guideline of azithromycin or moxifloxacin is recommended in light of Mycoplasma genitalium isolation (B-II).
- 3.
The patient's sexual partners of the previous 2 months should be assessed and treated with the same regimen as the patient. Sexual abstinence is advised until the end of the treatment or after the symptoms have disappeared (C-III).
Proctitis is defined as an inflammation of the rectum, proctocolitis rectum/colon and enteritis inflammation of the small intestine.
The patient may not show any symptoms until reporting symptoms of acute proctitis, such as anorectal pain, rectal tenesmus, mucous/purulent and/or bloody rectal discharge and, sometimes fever and/or general malaise.
The examination may show ulcers in the perianal region or the anal canal, edema and erythema of the rectal mucosa and mucopurulent and/or bloody rectal discharge.
Proctocolitis is presented as bloody diarrhea, abdominal pain, accompanied by fever and general malaise. With clinical symptoms as proctitis but affecting the colon (until the sigmoid rectal junction).
Enteritis is characterized by nausea and vomiting, diarrhea, abdominal distension, flatulence, weight loss and fever. Rectal mucosa does not present inflammatory signs to examination.
Empirical treatment with doxycycline 100mg 1 tablet every 12hours for 7 days and ceftriaxone 500mg IM should be used in patients with acute proctitis symptoms and signs or in the absence of microbiological results. If herpes virus or syphilis is suspected, give also specific treatment.
In patients with proctocolitis symptoms and who have recently traveled to geographical regions with amoebic dysentery prevalence (or who have maintained sexual relations with people from these regions) treatment with Metronidazole 500–750mg/every 8hours for 7–10 days is indicated.
Recommendations- 1.
It is recommended to collect samples for rectal discharge through examination, preferably performed with a cold-light proctoscope, in patients with clinical or epidemiological suspicion of proctitis or proctocolitis. In case of observing signs of acute proctitis, empirical treatment could be started while microbiological results are pending (B-III).
- 2.
It is recommended to perform a screening of other STD, because they frequently present with other concomitant STD (A-II).
Vulvovaginitis are characterized by secretion, discomfort and vulvar or vaginal itching, frequently being asymptomatic.
Bacterial vaginosisAlthough not considered an STD, it has been associated to an increase in the risk of infection and transmission of Chlamydia trachomatis, Neisseria gonorrhoeae or even type-2 HSV and HIV.
Its clinical diagnosis is possible by fulfilling three of Amsel diagnostic criteria (distinctive vaginal discharge, amine-like odor after treatment with KOH, clue cells in microscopy, or vaginal pH >4.5). The technique of choice for its laboratory diagnosis will be the concentration of Lactobacillus and characteristic microorganisms in the Gram.
The treatment of choice will be Metronidazole 500mg/12h by oral way for 7 days or 0.75% topical 5gr/24h, for 5 days.
Candidal vaginosisIt can be caused by C. albicans (80–92%) or candida non-albicans, with a growing incidence in recent years. Diagnosis is usually clinical and made by gynecological examination, being it possible to observe hyphae with KOH in the microscopy of vaginal discharge. The culture is recommended for diagnosis confirmation and, in case of complicated candidiasis, to identify possible azoles resistance.
Vaginal trichomoniasisThe infection by T. vaginalis is asymptomatic in at least 50% of women and 80% of men, with an almost exclusive sexual transmission etiology. Characteristically, vaginal trichomoniasis is presented as foul-smelling discharge with vulvar irritation that increases during menstruation, the typical sign of “strawberry cervix” in the examination.
In recent years, nucleic acids detection tests have become the technique of choice. These can be performed in vaginal discharges, endocervical or urethral discharges and urine.
Trichomoniasis treatment is recommended for symptomatic and asymptomatic patients, and for their sexual partners. Metronidazole or Tinidazole 2g one-dose by oral way in HIV-negative patients, Metronidazole 2c one-dose by oral way in pregnant women, and extended guidelines (Metronidazol 500mg 1 tablet/12h by oral way for 7 days) in HIV-positive patients and after treatment failures, whose main cause will be reinfection and lack of adherence to the treatment. Sexual abstinence is encouraged until 7 days after taking the antibiotics.
Recommendations- 1.
When bacterial vaginosis is diagnosed, treatment will be indicated to asymptomatic patients and to those who will be subject to an obstetrical or gynecologic procedure (B-II). Some experts recommend treatment in patients with unsafe sexual practices, even if they are asymptomatic (C-III).
- 2.
The treatment for candidal vulvovaginitis will only be indicated in symptomatic women. It is not routinely recommended for their sexual partners (A-I).
- 3.
Trichomoniasis treatment is recommended for symptomatic or asymptomatic patients, and their sexual partners. It is advised to perform a screening to rule out the coexistence of other STD (A-II).
Balanitis is an inflammatory process which affects the glans and usually coexists with an inflammation of the foreskin, called in this case balanoposthitis (BP).
Recommendations- 1.
Candida balanitis is not considered a sexually transmitted infection, so it is not necessary to study sexual contacts systematically. Each case should be individualized (C-III).
- 2.
It is recommended to pay special attention to persistent, recurrent or chronic balanitis. These types should be examined by a dermatology specialist (B-III).
Acute epididymo-orchitis is a clinical syndrome characterized by pain, edema and epididymis inflammation. The ipsilateral testicle may also be affected.
In sexually active patients younger than 35 years of age, the main microorganisms involved are sexually transmitted Chlamydia trachomatis, Neisseria gonorrhoeae and enterobacteriaceae if insertive anal sex has been practiced. In older groups, the case is frequently presented secondarily to complications in urinary infections, mainly by enterobacteriaceae and Pseudomonas aeruginosa.
Treatment should be offered empirically to all patients, depending on the etiologic suspicion: Ceftriaxone 500mg IM one-dose+Doxycycline 100mg 1 tablet/12h for 10–14 days. In case of suspicion of enteric organisms, Ciprofloxacin 500mg, 1 tablet/12h for 10 days.
Recommendations- 1.
Empirical treatment should be administered in case of clinical suspicion, without waiting for microbiological results (B-III).
- 2.
In patients under 35, the main suspicion should be the sexually transmitted etiology. Syndromic treatment is recommended (B-III).
- 3.
In patients with unprotected anal intercourse, apart from considering enteric organisms, microorganisms involved in the sexually transmitted etiology should be considered (B-II).
It is an acute infection of the internal genital tract that affects the uterus, the endometrium, the tubes or the ovaries and that may involve neighboring organs. A PID should be suspected in sexually active patients who present abdominal pain and pelvic discomfort.
If there is suspicion of PID, tests of cervical specimen should be performed to detect C. trachomatis and N. gonorroheae and of vaginal discharge to detect bacterial vaginosis. However, one should not wait for the results to establish the treatment.
Treatment is based on the combination of Ceftriaxone (500mg IM one-dose) plus doxycycline (100mg every 12h for 14 days by oral way)+/−metronidazole (500mg every 12h for 14 days by oral way); or Cefotaxime (1g, IM, one-dose) or Ceftizoxime (1g, IM, one-dose) plus doxycycline (100mg every 12h for 14 days by oral way)+/−metronidazole (500mg every 12h for 14 days by oral way).
Recommendations- 1.
Pelvic inflammatory disease should be suspected in every sexually active woman who presents pelvic pain or discomfort, especially if it is accompanied by fever or low-grade fever or other symptoms like vaginal discharge, urinary symptoms, intermenstrual or postcoital bleeding, etc. (A-II).
- 2.
The treatment of pelvic inflammatory disease is based on the administration of broad-spectrum antibiotics that encompass the most frequently involved germs (A-II). Treatment should be administered in hospitals in the case of certain risk factors, such as pregnancy, impairment in the general condition, presence of pelvic abscesses, etc. (A-II).
- 3.
HIV infection does not entail any differences in the response to the treatment of pelvic inflammatory disease. Therefore, its management is similar to that of non-infected patients (B-II).
Genital ulcers may have infectious or non-infectious causes, being sexually transmitted infections the most frequent ones. The presence of genital ulcers is a risk factor in HIV transmission.
Herpes simplex virusA relapsing or recurrent infection that may be caused by the two different herpes virus serotypes (HSV 1 and 2). Even though 30% of the first episodes of genital herpes are caused by HSV-1, relapses are much more frequent for HSV-2 in the genital area.
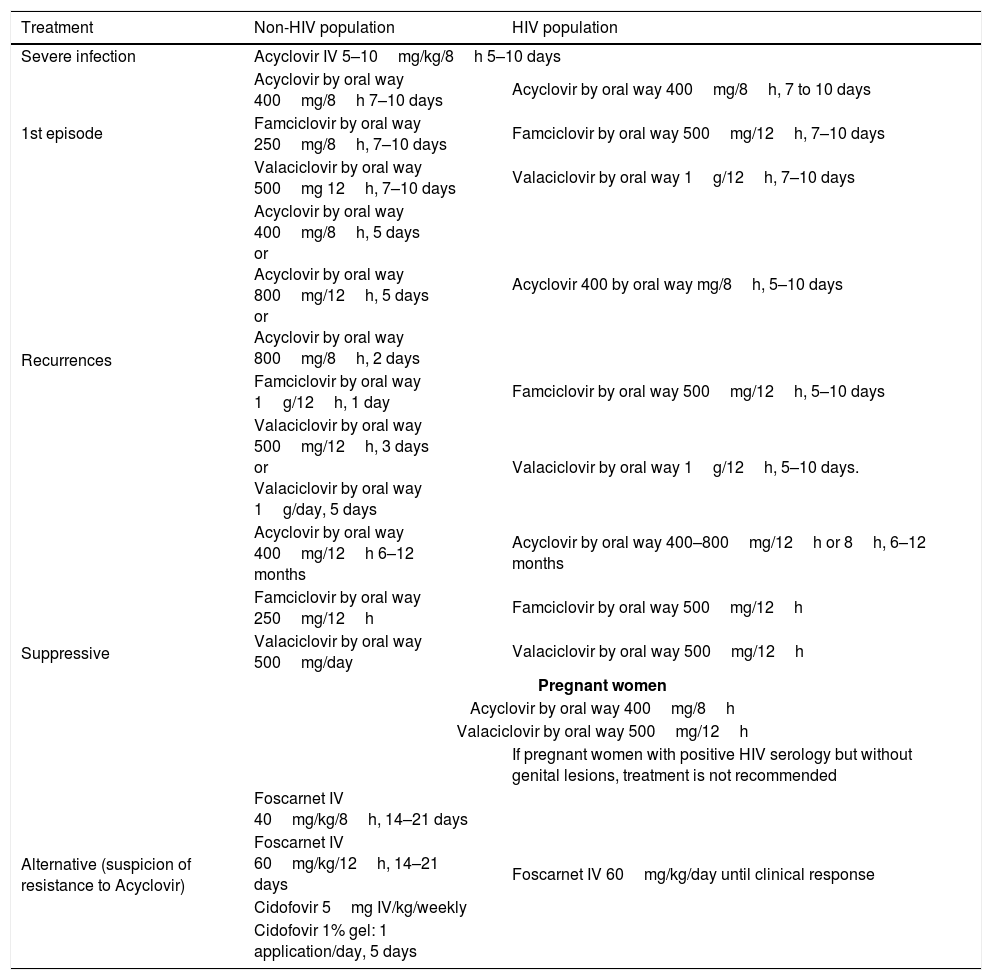
The treatment of relapsing genital herpes should start on the first day or in the prodromal period. Suppressive therapy reduces the frequency of relapses between 70 and 80% and many patients do not even present symptoms. Nevertheless, it is not so effective when it comes to reducing asymptomatic viral excretion. (Table 2)
Genital herpes treatment.
| Treatment | Non-HIV population | HIV population |
|---|---|---|
| Severe infection | Acyclovir IV 5–10mg/kg/8h 5–10 days | |
| 1st episode | Acyclovir by oral way 400mg/8h 7–10 days | Acyclovir by oral way 400mg/8h, 7 to 10 days |
| Famciclovir by oral way 250mg/8h, 7–10 days | Famciclovir by oral way 500mg/12h, 7–10 days | |
| Valaciclovir by oral way 500mg 12h, 7–10 days | Valaciclovir by oral way 1g/12h, 7–10 days | |
| Recurrences | Acyclovir by oral way 400mg/8h, 5 days or Acyclovir by oral way 800mg/12h, 5 days or Acyclovir by oral way 800mg/8h, 2 days | Acyclovir 400 by oral way mg/8h, 5–10 days |
| Famciclovir by oral way 1g/12h, 1 day | Famciclovir by oral way 500mg/12h, 5–10 days | |
| Valaciclovir by oral way 500mg/12h, 3 days or Valaciclovir by oral way 1g/day, 5 days | Valaciclovir by oral way 1g/12h, 5–10 days. | |
| Suppressive | Acyclovir by oral way 400mg/12h 6–12 months | Acyclovir by oral way 400–800mg/12h or 8h, 6–12 months |
| Famciclovir by oral way 250mg/12h | Famciclovir by oral way 500mg/12h | |
| Valaciclovir by oral way 500mg/day | Valaciclovir by oral way 500mg/12h | |
| Pregnant women | ||
| Acyclovir by oral way 400mg/8h | ||
| Valaciclovir by oral way 500mg/12h | ||
| If pregnant women with positive HIV serology but without genital lesions, treatment is not recommended | ||
| Alternative (suspicion of resistance to Acyclovir) | Foscarnet IV 40mg/kg/8h, 14–21 days | Foscarnet IV 60mg/kg/day until clinical response |
| Foscarnet IV 60mg/kg/12h, 14–21 days | ||
| Cidofovir 5mg IV/kg/weekly | ||
| Cidofovir 1% gel: 1 application/day, 5 days | ||
- 1.
In first-case patients, it is recommended to determine whether it is HSV-1 or HSV-2 for its management and guidance (B-III).
- 2.
PCR detection of HSV-DNA is the method of choice for diagnosis (A-I).
- 3.
When symptoms are suggestive, it is recommended to repeat the serology to demonstrate IgM seroconversion, specifically in cases with negative IgG (B-II).
- 4.
In suppressive treatment, Acyclovir is the therapy with most experience (A-I). There is no difference between Valaciclovir and Famciclovir (C-III).
It is infrequent in Europe but it is present in the Caribbean, South America, India and Africa. It is presented as a single or multiple painful ulcers and may be accompanied by regional lymphadenopathy.
Recommendations- 1.
Diagnosis is based on PCR techniques, with the advantage of being able to combine it with other pathogens such as T. pallidum and herpes virus (B-III).
- 2.
Sexual abstinence is recommended until the patient's partner has completed the treatment (C-III).
- 3.
First-line treatment: Ceftriaxone 250mg IM (A-I), azythromycin 1g one-dose (A-I).
- 4.
Second-line treatment: Ciprofloxacin 500mg BID for 3 days (B-I).
Primary lesion occurs, in the form of a painless pustule that ulcerates, at the inoculation site 3 to 30 days after contact. The secondary stage appears 3 to 6 months later, as inguinal or anogenitorectal syndrome accompanied by unilateral lymph node enlargements and fever, arthromyalgia and general malaise. In homosexual patients, proctitis and proctocolitis may be similar to Crohn's disease. In rare cases, it may advance into tertiary stage with chronic inflammatory response. Diagnosis is made by PCR.
Recommendations- 1.
Systematic study of LGV is recommended for all MSM who present receptive anal intercourse in the previous 6 months (A-II).
- 2.
There is a significant association between HIV and LGV (A-I).
- 3.
The treatment of choice is doxycycline 100mg every 12hours for 21 days (B-II).
- 4.
The alternative treatment is erythromycin 500mg every 6hours for 21 days or azithromycin 1g every week for 3 weeks (B-III).
Endemic disease from South Africa, India and Australia and unusual in the western world. It produces progressive ulcerative, bloody, painless lesions without regional adenopathy. Diagnosis: viewing of Donovan bodies in tissue samples.
Recommendations- 1.
Treatment of choice: azithromycin 1g once a week or 500mg every 24h for at least 3 weeks (B-I).
- 2.
Alternative treatment: doxycyline 100mg every 12h (C-III) or trimethoprim-sulfamethoxadole 160/800mg every 12h (C-III) for at least 21 days.
Primary syphilis is characterized by the presence of ulcers and adenopathy. The HIV infection has a very small influence on the symptoms, with multiple ulcers being observed more frequently.
Diagnosis is usually indirect, by serology (treponemal/non-treponemal). Some laboratories may perform direct diagnosis (dark field, direct fluorescent antibody test or PCR), which allows for immediate diagnosis, even before seroconversion. As a general rule, diagnosis is made following the same criteria for an HIV-negative patient and an HIV-positive one; however, the latter may present abnormal serologic reactions (false negatives, slow seroconversions or extremely high antibody titers).
Tracking of the response to the treatment is made by monitoring VDLR/RPR titer. The same technique should always be used and, ideally, in the same laboratory.
Neurosyphilis requires a CSF study. The test of choice is VDLR. When its result is positive, it indicates neurosyphilis; however, a negative result does not rule it out.
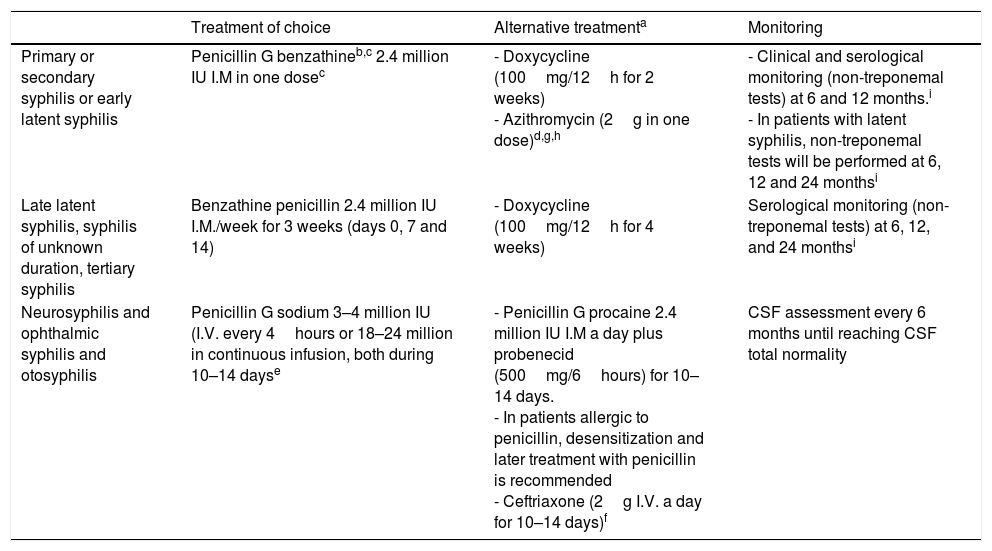
HIV patients should be treated with the same therapeutic regime as HIV-negative patients (Table 3).
Therapeutic recommendations for the treatment and monitoring of syphilis.
| Treatment of choice | Alternative treatmenta | Monitoring | |
|---|---|---|---|
| Primary or secondary syphilis or early latent syphilis | Penicillin G benzathineb,c 2.4 million IU I.M in one dosec | - Doxycycline (100mg/12h for 2 weeks) - Azithromycin (2g in one dose)d,g,h | - Clinical and serological monitoring (non-treponemal tests) at 6 and 12 months.i - In patients with latent syphilis, non-treponemal tests will be performed at 6, 12 and 24 monthsi |
| Late latent syphilis, syphilis of unknown duration, tertiary syphilis | Benzathine penicillin 2.4 million IU I.M./week for 3 weeks (days 0, 7 and 14) | - Doxycycline (100mg/12h for 4 weeks) | Serological monitoring (non-treponemal tests) at 6, 12, and 24 monthsi |
| Neurosyphilis and ophthalmic syphilis and otosyphilis | Penicillin G sodium 3–4 million IU (I.V. every 4hours or 18–24 million in continuous infusion, both during 10–14 dayse | - Penicillin G procaine 2.4 million IU I.M a day plus probenecid (500mg/6hours) for 10–14 days. - In patients allergic to penicillin, desensitization and later treatment with penicillin is recommended - Ceftriaxone (2g I.V. a day for 10–14 days)f | CSF assessment every 6 months until reaching CSF total normality |
In patients allergic to penicillin, alternatives have not been well-evaluated as to be able to recommend them as first-line therapy.
Although the majority of HIV patients respond to this treatment, some authors recommend adding 2 additional weekly doses of penicillin G benzathine 2,400,000IU I.M.
Azithromycin may be a useful therapeutic option for the treatment of primary and secondary syphilis. However, an increase in intrinsic resistance to this antibiotic and the existence of therapeutic failures has been observed.
Some authors recommend, once the previous treatment has been finalized, administering a weekly dose of penicillin benzathine 2,400,000IU I.M. for 3 weeks.
Chromosome mutations associated to the resistance to azithromycin and other macrolides have been detected. Therefore, azithromycin should not be used as a first-line drug, and in case it needs to be used, it will be used with caution, only when penicillin or doxycycline cannot be used.
- 1.
Whenever it is possible, it is recommended to perform a dark-field microscopy in chancre (A-II).
- 2.
PCR is a diagnostic tool which is useful in ulcers when serology is still negative. Its value is higher in diagnosis confirmation than in its exclusion (A-I).
- 3.
Serum with a positive screening test should be titrated through a non-treponemic VDLR/RPR test (A-I).
- 4.
Patients with HIV infection should be treated with the same therapeutic regime as HIV-negative patients, penicillin being the treatment of choice (A-II).
- 5.
In pregnant women, penicillin is the treatment of choice, even if they are allergic. Therefore, if necessary, it would be required to perform a desensitization and a treatment with penicillin (A-III).
- 6.
Azithromycin is not indicated to treat primary or secondary syphilis nor in HIV-positive patients (A-II).
- 7.
Lumbar puncture is indicated in every patient with a neurologic case (signs and/or symptoms) and/or treatment failure, to rule out the possibility of neurosyphilis (A-III).
- 8.
A screening test should be performed on all patients with syphilis to rule out other STD, including HIV (A-I).
- 9.
In sexually active patients (especially men who have sex with men and people with HIV) it is recommended to perform detection tests at least annually (A-I).
HPV infection is the most common STD in the world; up to three quarters of the world's population will get infected by HPV at some point. HIV-infected patients have a higher prevalence of HPV infection, together with a decrease in virus clearance by immunosuppression.
Liquid environment cytology is the test used for cervical and anal dysplasia screening, due to its low cost and its simple conducting. Because of cytology's variable sensitivity, certain authors suggest associating HPV-RA's PCR to the cytology in anal screening in case the cytology is normal. By doing this, we are able to increase diagnostic sensitivity for AIN2+ in HIV-positive patients MSM, in HIV-negative patients with cytology of uncertain significance, in HIV-negative MSM and in the case of cervical pathology in women with ASCUS, regardless of their age. In respect of HSIL/CA screening, the recommendation is: perianal area viewing and blind cytology and rectal exam; HRA in cases of cytology dysplasia, to locate lesions and take biopsy/ies and decide the approach to adopt.
There are different alternatives in the treatment of lesions produced by HPV in anal mucosa and in cervix/vagina/vulva which range from surgical excision, infrared coagulation, electrocautery with HRA, fulguration with CO2 laser, cryotherapy or medical treatments with Imiquimod, Trichloroacetic and bichloroacetic acid, Cidofovir or Podophyllotoxin solution at 0.5% or lotion 0.15%, and Sinecatechins for the treatment of external condyloma.
PreventionIn Spain, the immunization schedule considers vaccinating only girls, at 12 years of age (2 doses). There are populations with an increased risk of HPV pathology. These include HIV-infected, MSM or patients with chronic immunosuppressive treatment.
All this suggests that high-risk populations like MSM and people with HIV-infection could benefit from vaccination against HPV, until 26 years of age. Therefore, this Panel recommends reviewing the use of HPV vaccine in high-risk groups until 26 years of age, as different International Agencies also suggest.
Recommendations- 1.
Regarding HSIL/CA screening, 3 steps are recommended: perianal area viewing and blind cytology ans rectal exam; HRA in cases of cytology dysplasia, to locate lesions and take biopsy/ies and localized treatment and monitoring of HSIL lesions (B-II).
- 2.
The treatment of choice for external condyloma comprises Podophyllotoxin or Sinecatechins or Imiquimod (A-II).
- 3.
For the treatment of high-grade anal dysplasia, electrocoagulation/infrared light is generally recommended, even though there are different options, that may even be applied by the patient itself (B-II).
STD detection in children who have not yet started puberty requires an in-depth study, where sexual abuse should be ruled out first and in an exhaustive way, although other ways of transmission, such as perinatal or accidental, by auto- or hetero-inoculation, should be considered. In girls, endocervical samples are not usually taken, so vulvar or vaginal swabs should be sampled.
In case of sexual abuse or suspicion thereof, the intervention of members from attention mechanisms against violence or child abuse should be requested.
Generally, empirical treatment against STD is not recommended for all children, but may be considered in certain cases, depending on the risk of transmission and after the appropriate collection of microbiological samples has been performed. Post-exposure prophylaxis against HIV and HBV will be considered. The CDC also recommends starting or completing vaccination against HPV in these children from 9 years of age on.
AdolescentsAdolescents are more susceptible to the infection because of the immaturity of their genital tract. In sexually active female adolescents there is an increased risk of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) infection, and early exposure to HPV increases the risk of cervical dysplasia.
The study of certain STD (syphilis, trichomoniasis, bacterial vaginosis, HSV, HPV, HAV and HBV) is not recommended in asymptomatic adolescents, except in the case of MSM. The study of HIV and syphilis will be performed annually in risk populations.
Vaccination against HPV is recommended within the immunization schedule and before the initiation of sexual relations. Vaccination against HBV is recommended in case the patient has not already been vaccinated. HAV vaccination can be offered to adolescents and young adults who have not yet got it. It is indicated in risk groups (promiscuity, homosexuals, IDU).
Recommendations- 1.
STD detection in children who have not yet started puberty requires an in-depth study, where sexual abuse should be ruled out first and in an exhaustive way (A-II).
- 2.
In case of sexual abuse or suspicion thereof, adequate protection of the child, as well as appropriate notification of the case to judicial authorities, should be guaranteed (A-III).
- 3.
Healthcare professionals who take care of adolescents should integrate sexual education in their clinical practices. It is recommended to advise adolescents on sexual behavior associated to a higher risk of getting an STD and educate patients on risk-prevention strategies associated to them (A-II).
- 4.
Screening is recommended in case the patient's sexual history justifies it or when signs and symptoms appear that may be caused by an STD (B-II).
The preparation of this document has been financed with the funds from the SPNS (Spanish National AIDS Plan Secretariat).
Conflicts of interestsIn order to avoid and/or minimize any conflicts of interests, the individuals who make up this Expert Group have made a formal declaration of interests. In this declaration, some of the authors have received funding to take part in conferences and to conduct research, as well as having received payments as speakers for public institutions and pharmaceutical companies. These activities do not affect the clarity of the present document as the fees and/or grants received do enter into the recommended conflict of interests.
It must be pointed out, as regards the drugs in this document, that it only mentions the active ingredient and not the commercial brand.
The National Plan on AIDS and the Boards of the participating Scientific Societies, are grateful for the support and opinions of Emely García Carrasco, Gabriela Fagúndez, Saúl Barreales and Aurora Limia, that have contributed to improve the writing and enrich the contents of the document.
Rosa Polo. Médico especialista en Medicina Interna. Plan Nacional sobre el sida. Madrid. Rosario Palacios. Médico especialista en Medicina Interna. Unidad de Enfermedades Infecciosas. Hospital Virgen de la Victoria. Málaga. Antonia Andreu. Médico especialista en Microbiología. Hospital Universitario Vall d’Hebron. Barcelona José Luis Blanco. Médico especialista en Medicina Interna. Servicio de Enfermedades Infecciosas. Hospital Clínic, Barcelona. IDIBAPS. José Ramón Blanco. Médico especialista en Medicina Interna. Unidad de Enfermedades Infecciosas. Hospital San Pedro. CIBIR Logroño. Xavier Camino. Médico especialista en Medicina Interna. Consulta de ETS. Unidad de Enfermedades Infecciosas. Hospital Donostia. San Sebastián. Olivia Castillo. Médico especialista en Medicina Preventiva. Plan Nacional sobre el sida. Madrid. Adrià Curran. Médico especialista en Medicina Interna. Servicio de Enfermedades Infecciosas. Hospital Universitario Vall d’Hebron. Barcelona. Jorge del Romero. Mágister y Especialista Universitario en ETS. Unidad de ITS/VIH. Centro Sanitario Sandoval. Madrid. Asunción Díaz. Médico especialista en Medicina Preventiva y Salud Pública. Centro Nacional de Epidemiología. Instituto de Salud Carlos III/Plan Nacional sobre el sida. Madrid. Clotilde Fernández. Servicio de Microbiología. Hospital Universitario Puerta del Mar, Cádiz. Leire Gil. Médico especialista en Medicina Interna. Unidad de Hospitalización a Domicilio. Consulta de Infecciones de Transmisión Sexual (CAITS). Hospital Son Espases. Palma de Mallorca. Maribel Gonzalez Tomé. Médico especialista en Pediatría. Sección de Enfermedades Infecciosas Servicio de Pediatría. Hospital Universitario 12 de Octubre. Madrid. Carmen Hidalgo. Médico especialista en Medicina Interna. Unidad de Enfermedades Infecciosas. Hospital Universitario Virgen de las Nieves. Granada. Josefina López de Munain. Médico especialista en Medicina Familiar y Comunitaria y Medicina Preventiva y Salud Pública. Servicio de Enfermedades Infecciosas. Hospital Universitario Basurto. Bilbao. Nuria Margall. Médico especialista en Microbiología y Parasitologia. Hospital Universitario de la Santa Creu i Sant Pau. Marisa Navarro. Médico especialista en Pediatría. Sección de Enfermedades Infecciosas Servicio de Pediatría. Hospital Gregorio Marañón, Madrid. Luis Otero. Médico especialista en Microbiología Clínica. Servicio de Microbiología. Hospital de Cabueñes. Gijón. Asturias. Jose A Pérez-Molina. Médico Especialista en Microbiología Clínica. Servicio de Enfermedades Infecciosas. Hospital Universitario Ramón y Cajal. Madrid. Luis Prieto. Médico especialista en Pediatría. Servicio de Pediatría. Hospital de Getafe. Madrid. Eloy Muñoz. Médico especialista en Ginecología y Obstetricia. Hospital Universitario 12 de Octubre. Madrid. Teresa Puerta. Médico especialista en Dermatología. Unidad de ITS. Centro Sanitario Sandoval. Madrid. Isabel Pueyo. Médico especialista en Dermatología. Centro de ETS. Sevilla. Martí Vall. Médico especialista en Medicina Preventiva. Unidad de ITS, Programa Especial Enfermedades Infecciosas Vall d’Hebron-Drassanes. Barcelona. Fernando Vázquez. Médico especialista en Microbiología. Hospital Universitario Central de Asturias y Facultad de Medicina. Oviedo. Pompeyo Viciana. Médico especialista en Medicina Interna. Consultas externas de VIH. Servicio de Enfermedades Infecciosas Hospital Universitario Virgen del Rocío. Sevilla. Mª Carmen Viñuela. Médico especialista en Ginecología y Obstetricia. HGU Gregorio Marañon. Madrid. Mª Jesús Barberá. Médico especialista en Medicina Interna. Unidad de ITS, Programa Especial Enfermedades Infecciosas Vall d’Hebron-Drassanes. Hospital Universitario Vall d’Hebron. Barcelona.
See writing committee in Appendix A. All members of the panel are authors of this publication.
The entire version of the document can be found online as supplementary material in the journal official website (Appendix B).