To evaluate the efficiency of single-tablet regimens (STR) and multiple-tablet regimens (MTR) with exactly the same or different components.
MethodsA study was conducted on HIV-1-infected antiretroviral-naïve patients from 6 Spanish or French centers, who were started on treatment with STR-Atripla®, or the same components separately (MTR-SC), or a different MTR (MTR-Other). Effectiveness was measured as percentage of HIV-RNA <50copies/mL at 48 weeks (ITT). Efficiency was the ratio between costs (direct cost of antiretrovirals plus outpatient visits, hospital admissions, and resistance tests) and effectiveness.
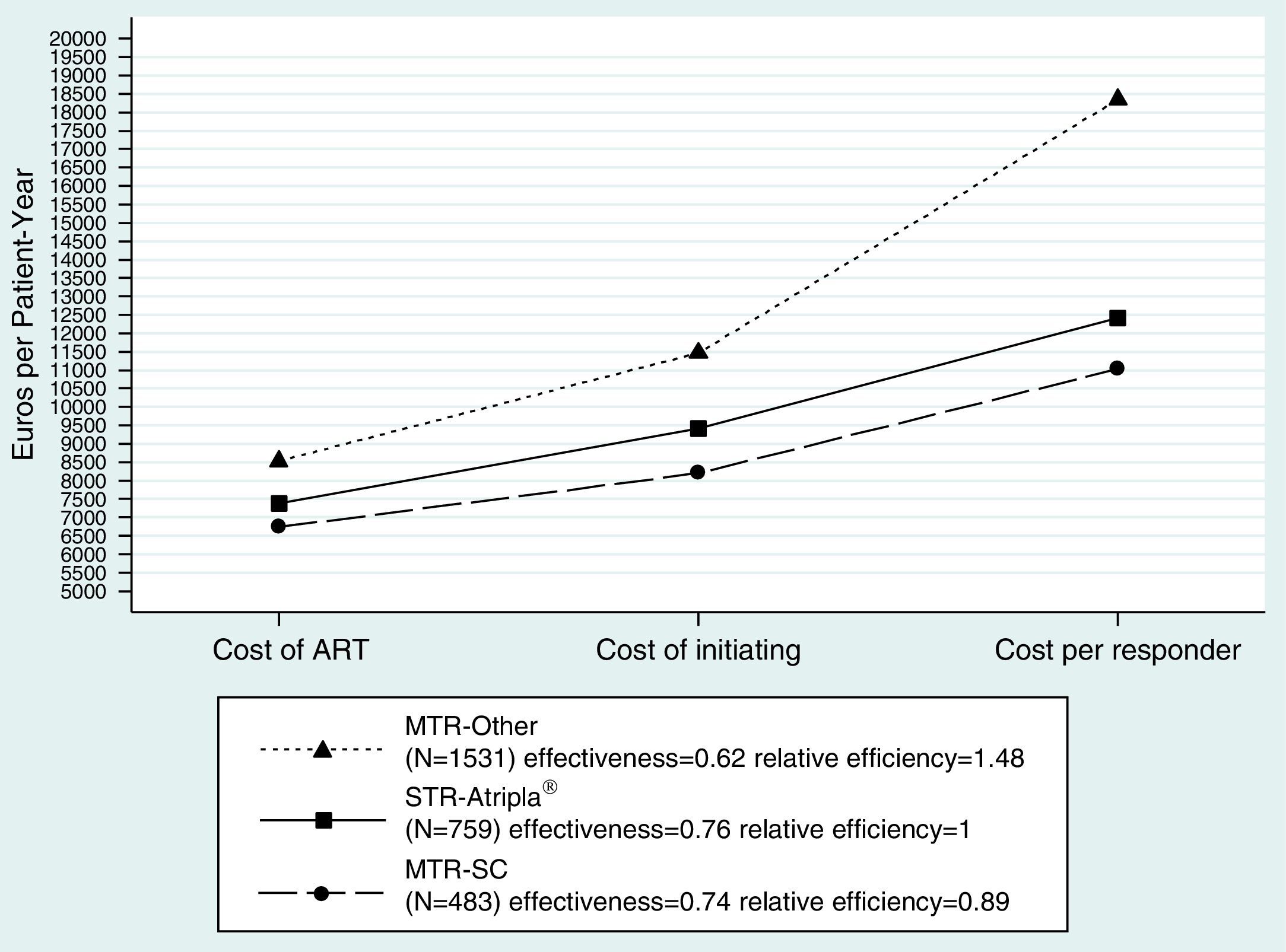
ResultsThe study included a total of 2773 patients (759 STR-Atripla®, 483 MTR-SC, and 1531 MTR-Other). Median age was 37 years, 15% were HCV co-infected, 27% had a CD4+ count <200cells/μL, and 30% had viral load ≥100.000copies/mL. The duration of the assigned treatment was longer for STR-Atripla® (P<.0001). Response rates (adjusted for CD4+ count, viral load, and clustered on hospitals) at 48 weeks were 76%, 74%, and 62%, respectively (P<.0001). Virological failure was more common in MTR patients (P=.0025), and interruptions due to intolerance with MTR-Other (P<.0001). Cost per responder at 48 weeks (efficiency) was €12,406 with STR-Atripla®, €11,034 with MTR-SC (0.89 [0.82, 0.99] times lower), and €18,353 (1.48 [1.38, 1.61] times higher) with MTR-Other.
ConclusionsSTR-Atripla® and MTR-SC regimens showed similar effectiveness, but virological failure rate was lower with STR-Atripla. MTR-SC, considered less convenient, had a marginally better efficiency, mainly due to lower direct costs. MTR-Other regimens had both a worse effectiveness and efficiency. Similar efficiency analyses adjusting for baseline characteristics should be recommended for new STRs.
Evaluar la eficiencia de un régimen antirretroviral de comprimido único diario (STR) y de regímenes de múltiples comprimidos (MTR) con exactamente los mismos (MTR-SC) o distintos componentes (MTR-Other).
MétodosSe incluyeron pacientes con infección por VIH-1 no tratados de 6 centros españoles o franceses que iniciaron tratamiento con STR-Atripla®, MTR-SC, o MTR-Other. La eficacia se midió como el porcentaje de VIH-ARN <50copias/ml (48 semanas, ITT). La eficiencia fue el cociente entre los costes (costes directos de los antirretrovirales, visitas ambulatorias, ingresos y estudios de resistencia) y la eficacia.
ResultadosFueron incluidos 2.773 pacientes (759 STR-Atripla®, 483 MTR-SC, 1.531 MTR-Other) con una edad media de 37 años, el 15% coinfectados por VHC, el 27% con CD4+ <200células/μl y el 30% con carga viral ≥100.000copias/ml. La duración del tratamiento asignado fue mayor para STR-Atripla® (p<0,0001). La respuesta (ajustada para CD4+, carga viral y centro hospitalario) a 48 semanas fue del 76, 74 y 62%, respectivamente (p<0,0001). El fracaso virológico fue más frecuente con ambos MTR (p=0,0025), y las interrupciones por intolerancia lo fueron con MTR-Other (p<0,0001). El coste por respondedor a 48 semanas (eficiencia) fue 12.406€ con STR-Atripla®, 11.034€ con MTR-SC (0,89 [0,82-0,99] veces menor), y 18.353€ (1,48 [1,38-1,61] veces mayor) con MTR-Other.
ConclusionesSTR-Atripla® y MTR-SC mostraron una eficacia similar, pero con menor fracaso virológico con STR-Atripla. MTR-SC, considerado menos conveniente, tuvo una eficiencia marginalmente mayor, principalmente debido a menores costes directos. MTR-Other tuvo una eficacia y eficiencia peores. Deberían recomendarse estudios similares con otros nuevos STR ajustados a las características basales de los pacientes.
Fixed-dose single tablet regimens (STR) are generally preferred by patients and physicians. As long as they include drugs of choice, co-formulated drugs are specifically recommended by antiretroviral treatment (ART) guidelines, unless dose-adjustments are required.1–4 They represent a significant advance in the simplification of ART, facilitating adherence to complex and chronic treatments, and contributing to a quantifiable improvement in patient's quality of life.5 STRs reduce the risk of treatment error, are associated with lower risks of hospitalization, can lessen the possibility of covert monotherapy if selective noncompliance, reduce the risk of developing HIV-1 resistance, and can impact treatment costs as well.6–13 One study found that STR led to a 17% reduction in overall healthcare costs.7 However, most of these studies have baseline prescription bias and no randomized clinical trials using drugs as they are prescribed in an unblinded way have demonstrated these potential benefits of STR so far. Moreover, efficiency of STR has never been compared with a MTR with same components.
We hypothesized that despite having a possible higher direct cost, the efficiency (cost–effectiveness) of STR might be better when compared with a multi-tablet regimen (MTR) with the same or different components in antiretroviral-naïve subjects.
MethodsThis was an observational open clinical study undertaken in a prospectively collected cohort data. All antiretroviral naïve HIV-infected patients from 6 reference centers (4 from Barcelona, Spain; 1 from Madrid, Spain; 1 from Paris, France) who initiated ART with the STR-Atripla®, with their exact components administered separately (Tenofovir disoproxil fumarate [DF]+Lamivudine/Emtricitabine+Efavirenz, or with Truvada®+Efavirenz; MTR-SC) or with a MTR with different components (MTR-Other) between June 2008 and December 2011 were eligible. ART was openly assigned by the treating physician. Effectiveness was measured as the percentage of individuals with plasma viral load <50copies/ml at 48 weeks using an ITT (missing or non-completer=failure) analysis. A switch from MTR-same components to STR-Atripla® in virologically suppressed patients was allowed if decided by the treating physician for simplification, and patients were censored. Tolerability was measured as percentage of interruptions of the assigned treatment due to side effects possibly or probably related to the study medication. Costs included the direct cost of antiretroviral medications (Hospital Clinic, Barcelona, April 2014) obtained from the laboratory sale price plus 4% VAT minus the 7.5% compulsory reduction required by the Spanish government. The payer perspective (National Health System) was applied considering only differential direct costs (official costs of the Catalan National Health System) for outpatient medical visits, hospital admissions, and genotypic resistance tests. Zidovudine and Lamivudine were already generics, the rest including Truvada® and Kivexa® are branded. Efficiency was calculated as the ratio between costs and effectiveness (quotient of the number of patients with undetectable viral load at week 48 [i.e. responders, numerator] and the number of patients initiated on ART [denominator]) for the base case scenario and for the most and less favorable scenarios in a sensitivity deterministic analysis. The central (base case) estimate of the cost and effectiveness was calculated using the mean of the costs and the mean of the effectiveness. The worst scenario was assessed using the lower 95% confidence limit of both estimates, and the best scenario using the upper 95% confidence limit. The cost of initiating a regimen comprises the cost of ART and all the consequences (i.e. adverse events or need to switch the regimen) incurred in 48 weeks due to the decision of initiating that regimen. Ethics Committee approval was obtained in the coordinating center and all data were anonymised for research analyses.
Statistical analysisBaseline characteristics were compared using χ2 test for categorical data and Kruskal–Wallis test for continuous data. Following the intent-to-treat (ITT missing or non-completer equals failure) principles, treatment failure at 48 week of follow-up was defined as: virological failure, treatment change or discontinuation whatever the reason, loss of follow-up or clinical progression to AIDS or death. Logistic regression model was used to estimate therapeutic efficacy in the three arms. Estimates were adjusted for baseline viral load (with cut-off 100,000) and CD4+ cells (with cut-off 200) and their standard errors were robust for clustering on hospitals. Time to treatment failure for the three groups was plotted using the Kaplan–Meier curves and the Cox regression model provided the hazard ratio (HR) adjusted for baseline viral load (with cut-off 100,000) and CD4+ (with cut-off 100,000) baseline with robust standard errors for clustering on hospitals. All tests were two-sided with a confidence level set to 5%. Stata software (StataCorp 2013, College Station, TX) was used for these analyses.
ResultsBaseline characteristicsA total of 2773 patients (759 (27%) STR-Atripla®, 483 (17%) MTR-SC, and 1531 (55%) MTR-Other) were included. There were significant differences among centers and between Spanish and French centers in the type of regimens prescribed, suggesting differences in local practices for ART prescription. Baseline characteristics are illustrated in the Supplemental Table 1. The most commonly prescribed MTR-Other regimens are listed in the Supplemental Table 2. The STR-Atripla® arm had a significantly higher percentage of males, homosexual male transmission, individuals with CD4+ cell count >200/mm3 (and a higher median CD4+ count) and higher median viral load compared with the MTR groups. The percentage of patients completing at least one year of follow-up was 98%, 97% and 94% in the STR-Atripla®, MTR-Same Components and MTR-Other, respectively (p=0.0001).
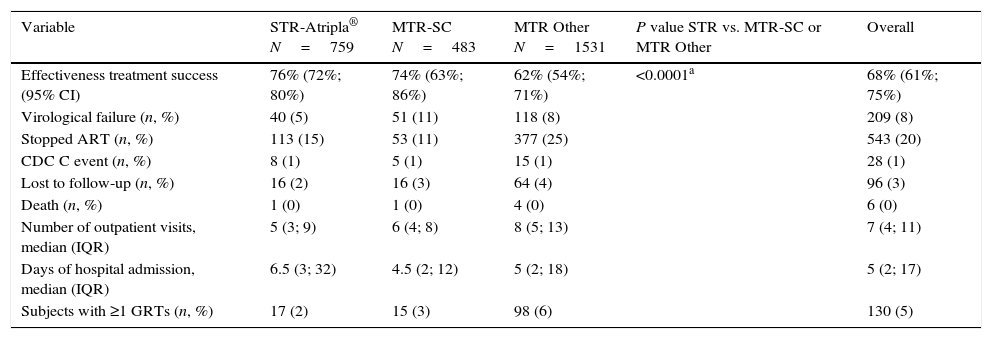
Efficacy outcomesThe duration of the initial assigned treatment regimen was significantly longer for the STR-Atripla® (p<0.0001). The adjusted estimates for response rates at 48 weeks were 76% (95% CI: 72; 80), 74% (95% CI: 63; 86) and 62% (95% CI: 54; 71) for STR-Atripla®, MTR-SC and MTR-Other, respectively (p<0.0001; Table 1). The analysis of effectiveness favored STR-Atripla® vs. MTR-Other (13.4%, 95% CI: 7.8, 19.1) but was similar between STR-Atripla® and MTR-SC 1.6% (95% CI: −8.8, 12.0). Virological failure was significantly more frequent in the MTR-SC and MTR-Other arms (p=0.0025) and specifically in MTR-sc (p=0.0005) vs. STR-Atripla®. Regimen withdrawal due to intolerance was more common among subjects receiving an MTR-Other regimen. Regimen withdrawal for any reason occurred in 15% of STR, 11% with MTR-SC, and 25% with MTR-other (p<0.0001). Progression to AIDS-defining events was uncommon, without differences among groups. Subjects lost to follow-up were evenly distributed among arms. Time to treatment failure was similar between STR-Atripla® and MTR-SC, and significantly longer with both regimens compared with MTR-Other (p<0.0001) (Suppl. Fig. 1). The hazards of treatment failure for STR-Atripla® and MTR-SC showed no significant differences (HR=0.98; 95% CI: 0.67, 1.43), and were higher in MTR-Other relative to STR-Atripla® patients with similar plasma HIV-1 RNA and CD4+ cell count at baseline (HR=1.59, 95% CI: 1.25, 2.01, p<0.0001).
Outcomes at week 48 (snapshot ITT M/NC=F analysis).
| Variable | STR-Atripla® N=759 | MTR-SC N=483 | MTR Other N=1531 | P value STR vs. MTR-SC or MTR Other | Overall |
|---|---|---|---|---|---|
| Effectiveness treatment success (95% CI) | 76% (72%; 80%) | 74% (63%; 86%) | 62% (54%; 71%) | <0.0001a | 68% (61%; 75%) |
| Virological failure (n, %) | 40 (5) | 51 (11) | 118 (8) | 209 (8) | |
| Stopped ART (n, %) | 113 (15) | 53 (11) | 377 (25) | 543 (20) | |
| CDC C event (n, %) | 8 (1) | 5 (1) | 15 (1) | 28 (1) | |
| Lost to follow-up (n, %) | 16 (2) | 16 (3) | 64 (4) | 96 (3) | |
| Death (n, %) | 1 (0) | 1 (0) | 4 (0) | 6 (0) | |
| Number of outpatient visits, median (IQR) | 5 (3; 9) | 6 (4; 8) | 8 (5; 13) | 7 (4; 11) | |
| Days of hospital admission, median (IQR) | 6.5 (3; 32) | 4.5 (2; 12) | 5 (2; 18) | 5 (2; 17) | |
| Subjects with ≥1 GRTs (n, %) | 17 (2) | 15 (3) | 98 (6) | 130 (5) |
STR: single-tablet regimen; MTR: multi-tablet regimen; MTR-SC: multi-tablet regimen with same components as Atripla®; MTR Other: multi-tablet regimen with other drugs different than Atripla®. GRT: genotypic resistance tests.
The cost per responder at 48 weeks (efficiency) in the base case scenario was 12,406€ in the STR-Atripla® arm, 11,034€ (0.89 [0.82, 0.99] relative cost/effectiveness vs. STR-Atripla®) in the MTR-SC, and 18,353€ (1.48 [1.38, 1.61]) in the MTR-Other. Similar trends were observed in the less and most favorable scenarios (Suppl. Table 3, Fig. 1).
Cost analysis of the base case scenario. Cost of ART: Drug costs of each regimen for 48 weeks (laboratory sale price+4% VAT−7.5% compulsory government reduction). Cost of initiating: cost of initiating an ART regimen including ART cost plus all potential consequences (adverse effects and changes to other regimens) that may occur within 48 weeks. Cost per Responder (efficiency): Cost of achieving one responder (plasma HIV-1 RNA <50copies/mL) by week 48 from the payer (Spanish NHS) perspective, calculated as the cost of initiating ART divided by its effectiveness. ART: antiretroviral therapy; STR: single tablet regimen; MTR: multiple tablet regimen; SC: same components as Atripla; Other: components other than those in Atripla®.
In this prospective multicenter study we did not found significant differences in the 48-week response rates, the median duration of assigned regimen, the rates of patients completing at least one year of follow-up, and the rates of interruptions for tolerance problems among subjects receiving their initial ART as STR-Atripla® or MTR-SC. However, the rates of virological failure were significantly lower with STR-Atripla®. The cost per responder (efficiency) at 48 weeks was lower for MTR-SC regimens. Those receiving MTR-Other regimens had lower effectiveness, higher rates of discontinuation, and a lower efficiency. In fact, this is the first study comparing the efficiency of a single tablet regimen with a multiple tablet regimen with same components using real data and not microsimulation models.14
We identified significant differences among centers and countries in the initial regimens prescribed. The most commonly MTR-Other regimens prescribed included a ritonavir-boosted protease inhibitor. Subjects in this arm had significantly lower CD4+ counts that those in the STR-Atripla® one, probably due to the perception among physicians of a higher efficacy of these regimens in this scenario, despite randomized clinical trials showing the opposite.15,16 The significantly longer durability of STR-Atripla® or MTR-SC seen in our series is in agreement with previous findings showing that, compared to efavirenz, patients on boosted protease inhibitors had higher rates of modification and interruption.17
The cost per responder at 48 weeks was lower for MTR-SC than for STR-Atripla® or MTR-Other, and this difference was driven but its lower costs. This is the first time this analysis has been done using prospective real-life data. These data should be taken into account by payer health agencies and policy managers when designing cost-saving strategies.12 Non-nucleoside reverse transcriptase inhibitor-based regimens (mainly efavirenz) have usually been considered cost-saving interventions.18–20
The significant differences seen in the adjusted estimates for response rates, with higher efficacy for STR-Atripla® or MRT-SC regimens will be particularly relevant in developing countries where this STR could be substituted by a MTR including generic components or to allow the use of a lower dose (400mg/day) of efavirenz.18,21 Unlike previous mathematical simulation models, our data have been captured from a prospective multicenter cohort and therefore have a higher accuracy.14,22 Even slight reductions in drug costs can substantially affect worldwide treatment scale-up and savings related to antiretroviral drugs.
Efavirenz-based regimens have currently been downgraded from preferred to alternative in most guidelines from well-resourced countries due to lower efficacy than dolutegravir, increased toxicity versus raltegravir and rilpivirine, and increased risk of suicide.1–3,23–27 However, new STR options are now recommended as preferred initial options, including dolutegravir/abacavir/emtricitabine, rilpivirine/tenofovir DF/emtricitabine, and elvitegravir/cobicistat/tenofovir DF/emtricitabine. This cost-effectiveness analysis using real-life prospective data should be reproduced with these new STRs to confirm their potential benefit versus current MTR.
Our study has some limitations. Subjects were not randomly allocated in their initial treatment arm but instead, their treatment allocation was a physician's decision. Therefore a channeling prescription bias could exist. Indeed, there were significant differences in baseline characteristics among groups, some of them with known impact on the rates of treatment efficacy. The STR-Atripla® arm had significantly higher percentages of subjects with high CD4+ cell counts and high viral load, associated respectively with better and worse prognosis in treatment response. Subjects in the MTR-Other group might have received their treatment due to reasons considered as making the subjects not eligible for efavirenz, as considered by their treating physician. However, even though physician's preferences over one or another regimen in initial therapy could have an impact at a local level, the inclusion of 6 centers belonging to two different countries, the high number of subjects included in the series and the estimates clustered on sites as well would reduce this potential bias. Further on this, significant differences remained in the multivariate analysis adjusted for HIV-1 RNA and CD4+ cell counts at baseline.
In summary, STR-Atripla® and MTR-SC had similar effectiveness, with higher rates of virological failure seen with MTR-SC. MTR-SC had a more favorable efficiency (cost–effectiveness) due to its lower cost. Both had a significantly better efficiency than the MTR-Other. These data were robust and confirmed in sensitivity analyses. Our data further support the recommendation of STR-Atripla® or MTR-SC (less convenient but more efficient) regimens as opposed to MTR-Other (worse efficiency). A similar efficiency analysis including all the newly approved STRs would be welcome.
FundingSupported in part by an unrestricted grant from Gilead Sciences.
Conflicts of interestJosep M. Llibre has been a member of the speakers’ bureaus and received fees from lectures or for participating in advisory boards from Janssen-Cilag, Merck Sharp & Dohme, ViiV Healthcare, Gilead Sciences and Bristol Myers Squibb.
Arkaitz Imaz has received financial compensation for lectures, consultancies, or educational activities from Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dome, and ViiV Healthcare.
Daniel Podzamczer has received research grants and/or honoraria for advisories and/or conferences from Boehringer Ingelheim, GSK, Viiv, Pfizer, BMS, Abbott, Gilead, Janssen and Merck.
Bonaventura Clotet has been a member of the speakers’ bureaus and received fees from lectures or for participating in advisory boards from Janssen-Cilag, Merck Sharp & Dohme, ViiV Healthcare, Gilead Sciences and Bristol Myers Squibb.
Pere Domingo has been a member of the speakers’ bureaus and received fees from lectures or for participating in advisory boards from Abbvie, Janssen-Cilag, Merck Sharp & Dohme, ViiV Healthcare, Gilead Sciences and Bristol Myers Squibb. My institution has received research grants from Janssen-Cilag, Merck, ViiV Healthcare, Gilead Sciences and Abbvie.
Josep M. Gatell has been a member of the speakers’ bureaus and received fees from lectures or for participating in advisory boards from Janssen-Cilag, Merck, Sharp & Dohme, ViiV Healthcare, Gilead Sciences and Bristol Myers Squibb and Abbvie. His institution has received research grants from Janssen-Cilag, Merck, Sharp & Dohme, ViiV Healthcare, Gilead Sciences and Bristol Myers Squibb and Abbvie.
Presented in part at: 8th IAS Conference on HIV Pathogenesis, Treatment & Prevention, 19–22 July 2015, Vancouver, Canada. Abstract TUBEB264.