Our work describes the frequency of superinfections in COVID-19 ICU patients and identifies risk factors for its appearance. Second, we evaluated ICU length of stay, in-hospital mortality and analyzed a subgroup of multidrug-resistant microorganisms (MDROs) infections.
MethodsRetrospective study conducted between March and June 2020. Superinfections were defined as appeared ≥48h. Bacterial and fungal infections were included, and sources were ventilator-associated lower respiratory tract infection (VA-LRTI), primary bloodstream infection (BSI), secondary BSI, and urinary tract infection (UTI). We performed a univariate analysis and a multivariate analysis of the risk factors.
ResultsTwo-hundred thirteen patients were included. We documented 174 episodes in 95 (44.6%) patients: 78 VA-LRTI, 66 primary BSI, 9 secondary BSI and 21 UTI. MDROs caused 29.3% of the episodes. The median time from admission to the first episode was 18 days and was longer in MDROs than in non-MDROs (28 vs. 16 days, p<0.01). In multivariate analysis use of corticosteroids (OR 4.9, 95% CI 1.4–16.9, p 0.01), tocilizumab (OR 2.4, 95% CI 1.1–5.9, p 0.03) and broad-spectrum antibiotics within first 7 days of admission (OR 2.5, 95% CI 1.2–5.1, p<0.01) were associated with superinfections. Patients with superinfections presented respect to controls prolonged ICU stay (35 vs. 12 days, p<0.01) but not higher in-hospital mortality (45.3% vs. 39.7%, p 0.13).
ConclusionsSuperinfections in ICU patients are frequent in late course of admission. Corticosteroids, tocilizumab, and previous broad-spectrum antibiotics are identified as risk factors for its development.
Nuestro trabajo describe la frecuencia de sobreinfecciones en pacientes con COVID-19 en UCI e identifica factores de riesgo asociados con su aparición. Secundariamente, evaluamos la estancia en UCI, mortalidad intrahospitalaria y analizamos un subgrupo de infecciones causadas por microorganismos multirresistentes (MDR).
MétodosEstudio realizado entre marzo y junio de 2020. Definimos como sobreinfección a aquellas que aparecieron ≥48h del ingreso. Incluimos las causadas por bacterias y hongos y evaluamos la infección respiratoria asociada a la ventilación mecánica (IRAVM), bacteriemia primaria, bacteriemia secundaria e infección del tracto urinario. Se realizó un análisis multivariante de los factores de riesgo.
ResultadosIncluimos 213 pacientes, documentándose 174 episodios de sobreinfección en 95 casos (44,6%): IRAVM 78 episodios, bacteriemia primaria 66, bacteriemia secundaria 9 e ITU 21. Los MDR causaron el 29,3% de los episodios. La mediana de tiempo hasta el primer episodio fue de 18 días, siendo mayor en las causadas por MDR vs. no MDR (28 vs. 16, p<0,01). El análisis multivariante identificó la administración de corticoides (OR 4,9 IC 95% 1,4-16,9), tocilizumab (OR 2,4 IC 95% 1,1-5,9) y antibióticos de amplio espectro (OR 2,5 IC 95% 1,2-5,1) como factores de riesgo asociados. Los pacientes con sobreinfección presentaron una estancia en UCI más prolongada (35 vs. 12 días, p <0,01) pero no mayor mortalidad intrahospitalaria (45,3% vs. 39,7%, p 0,13).
ConclusionesLas sobreinfecciones en los pacientes con COVID-19 aparecen tardíamente. La administración de corticoides, tocilizumab y antibióticos de amplio espectro se asocia con su aparición.
Since December 2019, SARS-CoV-2 has expanded and COVID-19 was declared a pandemic by the WHO on 11th March 2020.1,2 Currently, corticosteroids are the mainstream treatment for severe COVID-19.3–5 The role of tocilizumab and other immunosuppressive agents is debated, but is being applied worldwide, more often in the intensive care unit (ICU) setting.6,7 In addition, many inpatients with COVID-19 have empirically prescribed broad-spectrum antibiotics to treat possible bacterial coinfections.8
Taking in to account these factors, it raises the question of how much superinfections will be expected, especially in the ICU setting, where patients are more susceptible to bacterial and fungal infections.9 Most prior publications have not designed specifically for ICU patients or have only evaluated some types of infections, such as COVID-19 Associated Pulmonary Aspergillosis (CAPA) syndrome.10–13
In this scenario, it is reasonable to predict an increase in the rate of superinfections or antimicrobial resistance. Furthermore, data on ICU patients, antimicrobial usage, and antimicrobial stewardship programs reported during the first COVID-19 waves are scarce.14
We aimed to describe superinfections in ICU patients with COVID-19 in a tertiary care hospital in Madrid and to assess its impact on the outcome. The primary objective was to determine the frequency of superinfections in patients admitted to the ICU and to identify the risk factors associated with their development. Secondary objectives included the assessment of the ICU length of stay and in-hospital mortality of patients with superinfections compared to patients without infections (control group) and the analysis of the subgroup of patients with MDROs infections.
Materials and methodsDesignRetrospective study of adult patients with confirmed RT-PCR SARS-CoV-2 infection who required ICU admission in the Gregorio Marañón University Hospital (HGUGM) in the first COVID-19 wave, defined from March to June 2020.
Until March 2020, the HGUGM was a 1200-bed-hospital with 72 adult ICU beds. Subsequently, other critical units (surgical ward, library, ambulatory major surgery unit, and post-anesthetic care unit) were implemented to treat an excess of critical patient admissions that required mechanical ventilation support and other critical complications derived from COVID-19. All adult patients admitted to different ICUs were included.
We analyzed the clinical, laboratory, and event-related variables. We registered all COVID-19 medications received according to the local treatment protocol which included lopinavir/ritonavir, hydroxychloroquine, azithromycin, ceftriaxone, remdesivir, tocilizumab, interferon beta, and corticosteroids. Anticoagulant therapy (low-molecular-weight heparin at a dose of 1mg/kg/12h or unfractionated heparin) was administered in cases in which venous thrombosis was confirmed and/or in whom D-dimer levels increased above 2000–3000ng/mL at the discretion of the prescribing physician. Data of cases were obtained from the Medical Record, the Electronic-prescribing System, and from the Microbiology Department database.
Definitions- -
Superinfections were defined as their appearance 48h after admission. Confirmed bacterial and/or fungal infections require the cultures of a pathogen microorganism. MICs were determined according to EUCAST (v10.0) breakpoints. We stratified superinfections in three periods from ICU admission: early (less than 7 days), intermediate (7–13 days), and late (≥14 days).
- -
Primary bloodstream infection (BSI) was defined according to CDC criteria.15 Briefly, we included episodes of catheter-related BSI and those due to coagulase-negative staphylococci (CoNS), Enterococcus spp. and Candida spp. when the source was unclear.
- -
Catheter-related bloodstream infection (CR-BSI): require that the same organism (and the same antibiotic susceptibility in CoNS) grown from at least 1 percutaneous blood culture and from a culture of the catheter tip, or that two blood samples be drawn (one from a catheter hub and the other from a peripheral vein) that presented with differential positive cultures ≥2h favoring the catheter blood culture.
- -
Secondary BSI: defined by the presence of any demonstrated source (urinary, abdominal, respiratory, or other) of the bacteremia.
- -
Ventilator-associated lower respiratory tract infection (VA-LRTI): defined according to ATS-IDSA criteria, in which confirmed infection required the presence in semiquantitative cultures of a pathogen microorganism in a lower respiratory tract sample.16
- -
COVID-19 associated pulmonary aspergillosis (CAPA): was considered to recently published criteria.17
- -
Urinary tract infection (UTI): requires the presence of ≥105UFC/mL of a bacteria in a urinary sample with clinical signs congruent with an infectious process. We also included episodes of candiduria if the patient received a specific treatment.
- -
Episode of infection: we differentiated episodes if at least ≥7 days occurred between two consecutive microbiological isolations, including the same or different microorganisms.
- -
Multidrug resistant microorganisms (MDROs): included methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), linezolid-resistant CoNS, extended-spectrum beta-lactamase (ESBL) gram-negative bacilli, carbapenem-resistant Enterobacterales (CRE), non-fermenting gram-negative bacilli (NF-GNB), and P. aeruginosa MDR or XDR as previously defined.18
- -
Immunosuppression: patients diagnosed with HIV, hematologic and solid organ transplantation, immunosuppression therapy, chronic corticosteroid therapy (≥1mg/kg/day prednisone for longer than one month or equivalent), and primary immunodeficiencies.
- -
Broad-spectrum antibiotics administered in the first 7 days of ICU admission: included patients who received at least one dose of any of the following antimicrobials: antipseudomonal cephalosporins, piperacillin-tazobactam and/or carbapenems. Third-generation cephalosporins were not included because they were considered a standard of care in our local protocol.
- -
SOFA score at ICU admission: based on the criteria of the International Sepsis Definitions Conference.19
We performed an analysis of superinfections according to patient, episode, source, and microorganism, and included an analysis of MDROs infections. The VA-LRTI incidence rate was calculated as episodes per 1000 days of mechanical ventilation (MV). The primary BSI incidence rate was estimated in episodes per 1000 days of ICU admission.
Quantitative variables were expressed as median with interquartile range (IQR). Qualitative variables were expressed as frequencies and percentages. Univariate analysis of data comparisons was performed using the unpaired t-test for normally distributed continuous variables or the Mann–Whitney test for non-normally distributed variables. Categorical variables were compared using the χ2 test or Fisher's exact test when the χ2 test was not appropriate. Adjusted odds ratios (ORs) were computed using logistic regression analysis. Stepwise logistic regression analysis included variables with a p-value <0.05, in the univariate analysis. All statistical analyses were performed using PASW Statistics 18 for Windows (SPSS Inc., Chicago, IL, USA).
Ethical issuesThe local institutional review boards and ethics committees approved the project with code number MICRO.HGUGM.2020-032.
ResultsFrom March to June 2020, 218 patients were admitted to the ICU. We excluded in the analysis four patients who were transferred to another institution and from whom no data were available regarding their outcomes. Another patient was excluded because SARS-CoV-2 infection was not confirmed. A total of 213 patients were included in the analysis. The median age was 61 years (IQR 52–71), and 110 (51.6%) were men. One hundred eighty-four patients (86.4%) required mechanical ventilation (MV), and 16.5% underwent renal replacement therapy. The median length of ICU stay was 19 days (IQR 11–37) and in-hospital mortality was 40.8%.
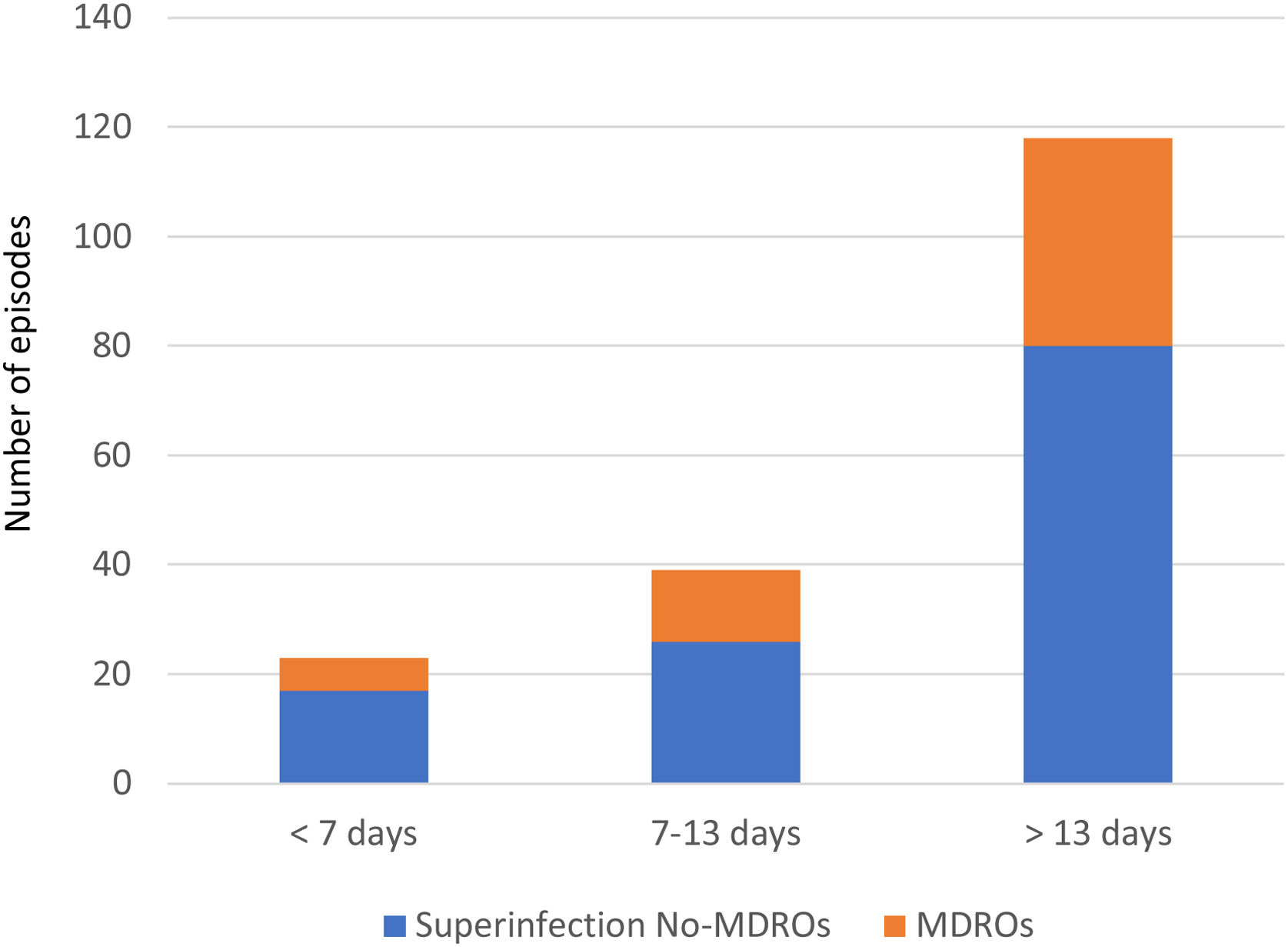
Overall, we documented 174 episodes of superinfections in 95 of 213 patients (44.6%) with an average of 1.83, superinfection episodes per infected patient. The median time from ICU admission to the first episode was 18 days (IQR 10–32). Taking into account the period in which superinfections appeared from ICU admission, in the early period superinfections represented 13.2% of the episodes, intermediate 22.4%, and late period 64.3% (Fig. 1).
Figure S-1 summarizes the source and the microbiology of the infections. Gram-positive bacteria caused 65 (37.4%) of the episodes, gram-negative bacilli 62 (35.6%), and Candida spp. 17 (9.7%). There were 78 episodes of VA-LRTI in 63 patients: 45 (57.6%) of the episodes were caused by gram-negative bacilli, in which the most frequent microorganisms isolated were NF-GNB (31 episodes), mostly caused by P. aeruginosa (17 episodes). In 6 cases Aspergillus spp. was the only microorganism isolated: four of these six cases were considered CAPA and patients received antifungal treatment. Sixty-six episodes of primary BSI were identified: 26 caused by CoNS, 15 Enterococcus spp., 10 Candida spp. (90% of the strains were C. albicans) and 5 episodes from B. cereus related to an outbreak in 2 of the ICU analyzed. Of the 66 primary BSI episodes, 42 (63.6%) were CR-BSI confirmed. Secondary BSI accounted for 9 episodes, in 5 of them GNB were isolated. There were 21 episodes of UTI: 8 episodes caused for Enterococcus spp., 4 Enterobacterales, 3 P. aeruginosa and 7 episodes of candiduria. VA-LRTI incidence rate was 17.2 episodes/1000 days of MV and primary BSI 13.1 episodes/1000 days of ICU admission. Levels of procalcitonin (PCT) and lactic acid for each type of infection are shown in Figure S-2.
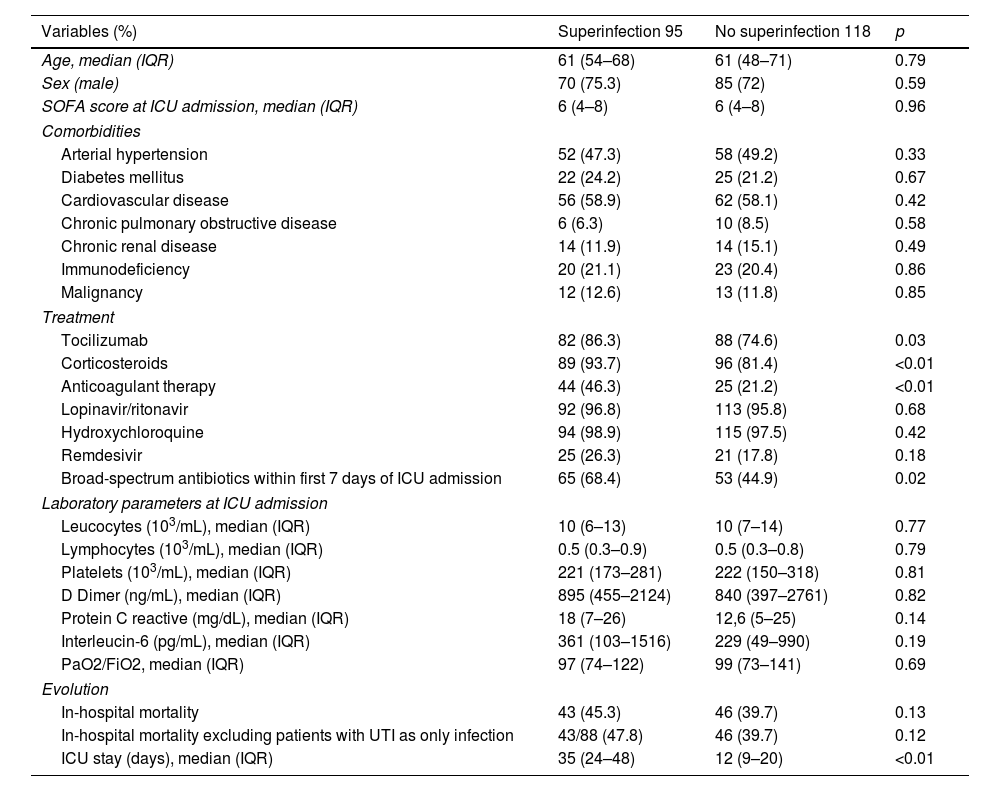
The clinical characteristics, laboratory values at ICU admission, treatments received, and evolution of the 213 patients are shown in Table 1. Patients with superinfections presented significantly respect to controls more frequent administration of tocilizumab (86.3% vs. 74.6%, p 0.03), corticosteroids (93.7% vs. 81.4%, p<0.01), anticoagulant therapy (46.3% vs. 21.2%, p<0.01), and broad-spectrum antibiotics in the first week of admission (68.4% vs. 44.9%, p 0.02).
Demographical, clinical and laboratory characteristics from the 213 patients.
| Variables (%) | Superinfection 95 | No superinfection 118 | p |
|---|---|---|---|
| Age, median (IQR) | 61 (54–68) | 61 (48–71) | 0.79 |
| Sex (male) | 70 (75.3) | 85 (72) | 0.59 |
| SOFA score at ICU admission, median (IQR) | 6 (4–8) | 6 (4–8) | 0.96 |
| Comorbidities | |||
| Arterial hypertension | 52 (47.3) | 58 (49.2) | 0.33 |
| Diabetes mellitus | 22 (24.2) | 25 (21.2) | 0.67 |
| Cardiovascular disease | 56 (58.9) | 62 (58.1) | 0.42 |
| Chronic pulmonary obstructive disease | 6 (6.3) | 10 (8.5) | 0.58 |
| Chronic renal disease | 14 (11.9) | 14 (15.1) | 0.49 |
| Immunodeficiency | 20 (21.1) | 23 (20.4) | 0.86 |
| Malignancy | 12 (12.6) | 13 (11.8) | 0.85 |
| Treatment | |||
| Tocilizumab | 82 (86.3) | 88 (74.6) | 0.03 |
| Corticosteroids | 89 (93.7) | 96 (81.4) | <0.01 |
| Anticoagulant therapy | 44 (46.3) | 25 (21.2) | <0.01 |
| Lopinavir/ritonavir | 92 (96.8) | 113 (95.8) | 0.68 |
| Hydroxychloroquine | 94 (98.9) | 115 (97.5) | 0.42 |
| Remdesivir | 25 (26.3) | 21 (17.8) | 0.18 |
| Broad-spectrum antibiotics within first 7 days of ICU admission | 65 (68.4) | 53 (44.9) | 0.02 |
| Laboratory parameters at ICU admission | |||
| Leucocytes (103/mL), median (IQR) | 10 (6–13) | 10 (7–14) | 0.77 |
| Lymphocytes (103/mL), median (IQR) | 0.5 (0.3–0.9) | 0.5 (0.3–0.8) | 0.79 |
| Platelets (103/mL), median (IQR) | 221 (173–281) | 222 (150–318) | 0.81 |
| D Dimer (ng/mL), median (IQR) | 895 (455–2124) | 840 (397–2761) | 0.82 |
| Protein C reactive (mg/dL), median (IQR) | 18 (7–26) | 12,6 (5–25) | 0.14 |
| Interleucin-6 (pg/mL), median (IQR) | 361 (103–1516) | 229 (49–990) | 0.19 |
| PaO2/FiO2, median (IQR) | 97 (74–122) | 99 (73–141) | 0.69 |
| Evolution | |||
| In-hospital mortality | 43 (45.3) | 46 (39.7) | 0.13 |
| In-hospital mortality excluding patients with UTI as only infection | 43/88 (47.8) | 46 (39.7) | 0.12 |
| ICU stay (days), median (IQR) | 35 (24–48) | 12 (9–20) | <0.01 |
Compared to the control group, patients with superinfections presented a prolonged ICU length of stay (35 vs. 12 days, p<0.01). There was a non-significant trend toward higher in-hospital mortality in the superinfection group (45.3% vs. 39.7%, p 0.13). After excluding seven patients in whom UTI was the only documented superinfection, in-hospital mortality rates were 47.8% in the superinfection group vs. 39.7% in controls, p 0.12. No significant differences were found in the remaining variables analyzed.
In the multivariate analysis (Table 2), factors independently associated with superinfections were corticosteroids (OR 4.9, 95% CI 1.4–16.9, p 0.01), tocilizumab (OR 2.4, 95% CI 1.1–5.9, p 0.03), broad-spectrum antibiotics within the first 7 days of ICU admission (OR 2.5, 95% CI 1.2–5.1, p<0.01), mechanical ventilation (OR 6.0, 95% CI 1.6–22.3, p<0.01), and anticoagulant therapy (OR 3.0, 95% CI 1.5–6.0, p<0.01).
Multivariate analysis of the factors associated with superinfections.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Mechanical ventilation | 6.0 | 1.6–22.3 | <0.01 |
| Tocilizumab | 2.4 | 1.1–5.9 | 0.03 |
| Corticosteroids | 4.9 | 1.4–16.9 | 0.01 |
| Broad-spectrum antibiotics within first 7 days of ICU admission | 2.5 | 1.2–5.1 | <0.01 |
| Anticoagulant therapy | 3.0 | 1.5–6.0 | <0.01 |
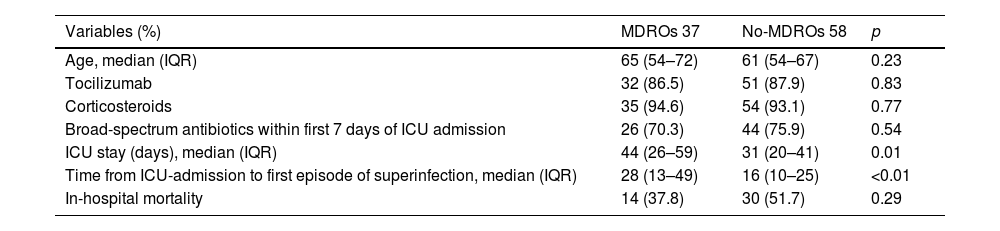
Thirty-seven of the 95 patients (38.9%) presented with 51 episodes of MDROs superinfection. The distribution in periods was: 11.7% in the early period, 13.7% in the intermediate and 74.5% in the late period from ICU admission (Fig. 1). In the comparison of the cases with MDROs (n=37) vs. patients with superinfections not caused by MDROs (n=58), patients with MDROs had a prolonged ICU stay (44 vs. 31 days, p<0.01). In-hospital mortality was not significant different between the two groups (37.8% vs. 51.7%, p 0.29). No differences were observed in the rate of corticosteroid use, tocilizumab or broad-spectrum antibiotics in the first week (Table 3).
Comparison between patients with MDROs vs. no-MDROs infections.
| Variables (%) | MDROs 37 | No-MDROs 58 | p |
|---|---|---|---|
| Age, median (IQR) | 65 (54–72) | 61 (54–67) | 0.23 |
| Tocilizumab | 32 (86.5) | 51 (87.9) | 0.83 |
| Corticosteroids | 35 (94.6) | 54 (93.1) | 0.77 |
| Broad-spectrum antibiotics within first 7 days of ICU admission | 26 (70.3) | 44 (75.9) | 0.54 |
| ICU stay (days), median (IQR) | 44 (26–59) | 31 (20–41) | 0.01 |
| Time from ICU-admission to first episode of superinfection, median (IQR) | 28 (13–49) | 16 (10–25) | <0.01 |
| In-hospital mortality | 14 (37.8) | 30 (51.7) | 0.29 |
MDROs caused 51 out of 174 episodes (29.3%). The time from ICU admission to the first episode was significantly longer in MDROs vs. no-MDROs episodes (28 vs. 16 days, p<0.01). Of the 51 MDROs episodes, 41 were due to GNB and 10 were due to gram-positive bacteria. The prevalence of MDROs in gram-positive bacteria was 30% for MRSA, 8.6% for VRE, and 18.5% for CoNS resistant to linezolid. In gram-negative bacteria, MDROs accounted for 63.5% of the episodes; 100% of the K. pneumoniae isolates were MDROs (10 cases: six ESBL, three OXA-48, and one ESBL+OXA-48), 40% of the E. coli were ESBL, and 50% of the P. aeruginosa isolates were MDR/XDR (none were carbapenemase-producing strains). Additionally, there were 17 episodes caused by other NF-GNB: B. cepacea in 9 episodes, S. maltophilia in 2 episodes, and other NF-GNB in the remaining episodes (Fig. 2).
DiscussionIn our study, superinfections occurred in 44.6% of COVID-19 patients admitted to the ICU, and MDROs accounted for 29.3% of the total episodes. We observed that infections occurred late in ICU hospitalization, with a prolonged period in those caused by MDROs. We identified corticosteroids, tocilizumab, and broad-spectrum antibiotics within the first week of admission independently associated with the development of superinfections. Finally, in-hospital mortality was not different between patients with and without superinfections, nor in the subgroup of MDROs vs. No-MDROs infections.
The rate of coinfections reported in hospitalized COVID-19 patients (including medical and ICU wards) ranges from 0.4% to 6.7%,10,20 which is lower than that in patients with influenza.21 These data do not support the routine use of antibiotics. In contrast, superinfections were more frequent during hospitalization. Previous studies have reported an incidence of superinfections in COVID-19 hospitalized patients ranged from 4.7% to 21.9%.10,11,22 In the ICU setting, data are more limited: there are publications that have focused on the source of infection, such as VA-LRTI or BSI,23–25 or on some specific microorganisms as Aspergillus spp.13 In our study, we evaluated all bacterial and fungal superinfections that appeared during ICU admission and analyzed them according to the source of the infection and the causative microorganisms with special consideration for MDROs infections.
We reported a 44.6% incidence of superinfection in patients admitted to the ICU. Our data contrast with previous reports in which the rates of superinfections were lower than ours. We believe that these differences could be because we analyzed only ICU patients, while other studies have also included patients admitted to medical wards, and for the period analyzed until a superinfection occurred was different in our work with respect to previous studies.10,11 We observed that superinfections developed with a median of 18 days from ICU admission and MDROs at 28 days. The appearance late in the course of hospitalization is in accordance with previous reports: MDROs infections occur in longer admissions, in MV patients and if broad-spectrum antibiotics are previously administered.11,26,27 Nevertheless, we cannot determine if superinfections were the cause or, by contrast, the consequence of prolonged ICU admission.
The incidence rate of VA-LRTI was 17.2 episodes/1000 days of MV, which contrast with previous data from our institution: in 2019 the incidence rate of VA-LRTI was 6.56 episodes/1000 days of MV, three times lower than in the first COVID-19 wave. However, our study was not designed to compare incidence rates of infection in ICU patients.
Risk factors independently associated with the development of a superinfection were corticosteroids OR 4.9 (95% CI 1.4–16.9, p 0.01), tocilizumab OR 2.4 (95% CI 1.1–5.9, p 0.03) and broad-spectrum antibiotics OR 2.5 (95% CI 1.2–5.1, p<0.01). However, all these risk factors could be associated with severe COVID-19 infections and reflect patients who are admitted to ICU wards for longer periods.
The in-hospital mortality rate in our series was 40.8%, which is in agreement with previous reports.28,29 We did not find a significant difference in mortality in patients who developed a superinfection episode, even after excluding patients in whom UTI was the only documented source. The reasons for our findings included that superinfections appeared late in the course of ICU admission, reflecting that most of the patients had survived the first few weeks of hospitalization. The infectious disease team that counseled in the ICU could also had a positive impact on patient prognosis.
The main limitation of our study was its retrospective design. We did not calculate the cumulative corticosteroid dose and the state of colonization for MDROs was not determined. Some of the UTI infections, especially those caused by Candida spp. could only represent colonization. Finally, our study included patients admitted during the first COVID-19 wave and treatment changes that occurred could not reflect the actual standard of care.
Strengths included the analysis of the total population admitted to the ICU in the first wave of COVID-19 in our hospital, the distribution of the source of the infection, the microorganisms involved and the identification of the risk factors associated with their development.
ConclusionsSuperinfections in COVID-19 patients admitted to the ICU are frequent but appear late in the course of admission. Corticosteroids, tocilizumab, and previous administration of broad-spectrum antibiotics have been identified as risk factors for its development.
FundingNone.
Conflicts of interestThe authors report no conflict of interest. All authors have submitted the ICMJE form for disclosure of potential conflicts of interest.
Members of Marañón Critical COVID-19 Infection Group: Alcalá (Luis), Aldámiz (Teresa), Almaraz (Carolina), Alonso (Roberto), Álvarez (Beatriz), Álvarez (Cristina), Álvarez-Uría (Ana), Arenal (Sara), Arias (Alexi), Arroyo (Luis Antonio), Barrios (Juan Camilo), Berenguer (Juan), Bermejo (Esther), Bermúdez (Elena), Bouza (Emilio), Burillo (Almudena), Butragueño (Laura), Calvo-García (Carlos Alberto), Candela (Ana), Cango (Nadia), Carrillo (Raquel), Casanova (Sara), Castañeda (Galo), Catalán (Pilar), Cercenado (Emilia), Cieza (Raquel), Cobos (Alejandro), Cui (Jie), De la Mata (Sara), Del Castillo (Jimena), Díez (Cristina), Elvira (Adoración), Erkicia (Iñaki), Escribano (Pilar), Estévez (Agustín), Fanciulli (Chiara), Fernández (Nerio), Fernández (Sarah), Fernández-López (Ignacio), Galar (Alicia), García (Mª Dolores), García-Bunguer (Beatriz), García de Viedma (Darío), Gijón (Paloma), Gómez (José Manuel), González (Adolfo), González (Rafael), Guerrero (José Eugenio), Guillén (Helmuth) Guinea (Jesús), Haces (Laura Vanessa), Herrera (Laura), Jardón (Eduardo), Jaspe (Alexis), Kestler (Martha), López (Juan Carlos), López-Gil (Elena), López-Morales (Sergio Benjamín), Losada (Carmen Narcisa), Machado (Marina), Manrique (Gema), Marín (Mercedes), Martín (Pablo), Mencía (Santiago), Montilla (Pedro), Moreno (Beatriz), Moure (Zaira), Oliver (Pablo), Olmedo (María), Palencia (María), Palomo (María), Parras (Francisco), Peral (José), Pérez-Granda (María Jesús), Pérez (Laura), Pérez (Leire), Pescador (Paula), Power (Mercedes), Ramirez (Carlos), Ramos-Cerro (Silvia), Ramos-Mejía (Santiago), Reigadas (Elena), Rincón (Cristina), Rodríguez (Belén), Rodríguez (Sara), Rojas (Adriana), Ruiz-Serrano (María Jesús), Sánchez (Amelia), Sánchez (Carlos), Sánchez (Mar), Sancho (Milagros), Santa-Teresa (Patricia), Santiago (María José), Sanz (Débora), Serrano (Julia), Slocker (María), Sevilla (Raúl), Solana (María José), Sotillo (Carlos), Tejerina (Francisco), Urbano (Javier), Valerio (Maricela), Veintimilla (Mª Cristina), Velasco-Rodrigo (Laura), Vesperinas (Lara), Vicente (Teresa).