Chronic diarrhea is defined as a diarrheal process that lasts more than 14 days and that may be associated, although not exclusively, with an infectious cause (persistent diarrhea). It is a relatively common problem in pediatric age, originating approximately 2–5% of pediatric consultations, and it is generally of functional origin.1,2
In this context, it is important to assess the inflammatory status and the quantification of fecal calprotectin is a useful, simple, and non-invasive test that can rule out intestinal inflammation in children with gastrointestinal symptoms. However, diagnostic accuracy of fecal calprotectin has not yet been adequately evaluated in pediatric persistent diarrhea.3
We report the diagnostic utility of fecal calprotectin in chronic diarrhea of bacterial etiology in pediatric patients. Seven hundred and seventy-seven patients (52.3% males, 47.7% females) with a diarrheal process lasting more than 14 days and aged between 1 and 14 years (median: 6 years) were included. This study met ethical recommendations of the Declaration of Helsinki4 and it was approved by the Sevilla Sur Research Ethics Committee (No.1569-M1-17).
Calprotectin was quantified by fluorescence enzyme immunoassay in a Phadia®100 analyzer (Thermo Fisher Scientific, Germany). A laboratory study of infectious etiology was carried out by molecular techniques (LightMix® Modular-Gastro-Bacteria-Parasite-Virus Multiplex Testing. Roche, USA). Data were processed using R-software (R-Core Team, 2014) and MedCalc 13.0 software (Ostend, Belgium).
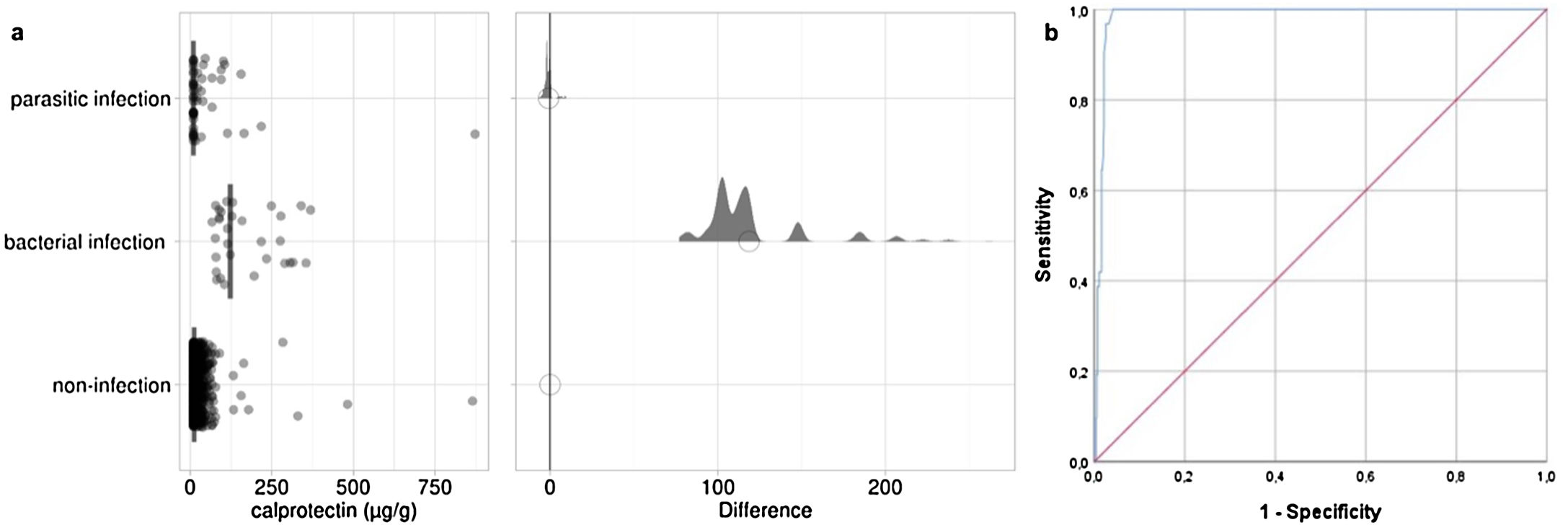
The results showed that in 11.3% of patients an infectious cause was detected and in 12.2% an inflammatory process was detected, although, in many cases, these processes were not isolated findings, but they were interrelated. Non-infectious causes were detected in 693 patients with a median calprotectin of 12μg/g (median absolute deviation (MAD): 2; confidence interval (CI) 95%: 10–14.2) (Fig. 1a). Regarding cases in which an infectious origin of diarrhea was found, bacteria of clinical interest were detected in 31 patients (4%) with a median calprotectin of 123μg/g (MAD: 44; CI95%: 95–218) (Fig. 1): the most prevalent bacteria were Campylobacter spp. with 20 cases (64.5%), followed by Salmonella spp. with 11 cases (35.5%). The presence of parasites was detected in 55 patients (7.1%) with a median calprotectin of 10μg/g (MAD: 0; CI95%: 10–19) (Fig. 1a); in 22 cases (40%) the parasites had a proven clinical value (Giardia spp., Cryptosporidium spp.) while in 33 cases (60%) the parasites were of uncertain clinical significance (Blastocystis spp., Dientamoeba spp.) (Fig. 1a). Viral etiology was not very prevalent, being detected in 2 patients (0.3%), and both corresponding to an infection by adenovirus. Furthermore, in 4 patients, there was a concomitant bacterial and parasitic infection: 2 cases of Giardia spp. and 1 case of Blastocystis spp. associated with diarrhea caused by Salmonella spp., and 1 case of Blastocystis spp. associated with diarrhea caused by Campylobacter spp.
Calprotectin results greater than 50μg/g have been observed in some patients with a parasitic infection and in some patients without an infection. These patients presented a concomitant bacterial infection, inflammatory bowel disease, hematochezia, processes associated with immunoglobulin A deficiency, or autoimmune disease. Specifically, in the hematochezia cases studied, the median calprotectin was 118.5μg/g; other recent studies show that calprotectin represents approximately 30% of neutrophil cytosol and, therefore, calprotectin levels increase as the extent of inflammation and bleeding increases.5
According to the results, the optimal cut-off point to differentiate bacterial diarrhea in the pediatric community was calculated in the ROC (receiver operating characteristic) curve. A calprotectin value of 74μg/g (Fig. 1b) presented an adequate discriminatory value with a sensitivity of 96.77%, specificity of 97.45%, positive predictive value of 80.6% and negative predictive value of 99.6%.
These results agree with other studies which indicate that fecal calprotectin may be a useful biomarker in children with infectious diarrhea, since calprotectin values increase in an acute bacterial infection and they are related to the severity of symptoms. In the case of chronic diarrhea, different studies have reported its utility in the evaluation and discrimination of infectious diarrhea of bacterial etiology in children.6 Usefulness of calprotectin in parasitic diarrhea in children has not been studied much, but the results of the only available study agree with the findings of our study: in absence of other associated underlying causes, the values of calprotectin in a parasitic infection are very low, except in an Entamoeba histolytica infection.7
In conclusion, our findings suggest the use of calprotectin as a biomarker of chronic diarrhea of bacterial etiology in pediatric patients. However, the discriminatory value of calprotectin can be affected in other situations such as hematochezia, inflammatory bowel disease, celiac disease, or the inclusion of children under 1 year of age. Therefore, their values should be interpreted in conjunction with clinical symptoms and other laboratory tests.