Frontline health care workers (HCW) have higher risk than the general population to become infected by SARS.CoV.2, so they were a priority group for Covid-19 vaccine administration. We compared the incidence and prevalence of HCW infected pre-and post-vaccination with the BNT162b2 mRNA COVID-19 vaccine.
Material and methodsProspective observational study carried out between 01/12/20 and 07/03/21 in La Paz University Hospital, Madrid (Spain). SARS.CoV.2 positive cases in HCW after vaccination were collected and compared to those hospitalized COVID-19 patients at the same hospital.
ResultsTwo weeks after finishing the first round of vaccinations daily new cases of HCW infections (symptomatic and asymptomatic) decreased substantially and cumulative cases of infected HCW and hospitalized COVID-19 patients started to diverge. No new positive cases of HCW infection were registered seven days after the second dose of BNT162b2 mRNA COVID-19 vaccine.
ConclusionsBNT162b2 mRNA COVID-19 vaccine is highly effective in Spanish HCW.
Los trabajadores sanitarios (TS) de primera línea tienen mayor riesgo de infectarse de SARS-CoV-2 que la población general, por lo que han sido un grupo prioritario para la vacunación frente a COVID-19. Comparamos la incidencia y prevalencia de TS infectados antes y después de la vacunación con BNT162b2 mRNA frente a COVID-19.
Material y métodosEstudio prospectivo observacional realizado entre 01 de diciembre de 2021 en el Hospital Universitario La Paz, Madrid, España. Se registraron los casos positivos para SARS-CoV-2 en TS y se compararon con los hospitalizados por COVID-19.
ResultadosDos semanas tras la primera ronda de vacunación las nuevas infecciones en TS (sintomáticas y asintomáticos) disminuyeron sustancialmente y los casos acumulados de TS infectados y pacientes hospitalizados por COVID-19 empezaron a divergir. No hubo nuevas infecciones en TS vacunados a los siete días de la segunda dosis de la vacuna.
ConclusiónLa vacuna BNT162b2 mRNA frente a SARS-CoV-2 es altamente eficaz en TS españoles.
On December 21st, 2020, the European Commission authorized BioNTech and Pfizer's BNT162b2 mRNA Covid-19 vaccine.1 Spain began vaccinating on Sunday 27th December prioritizing two groups: (1) Residents and staff working in long-term care facilities for the elderly or the highly dependent and (2) frontline staff in the health and social-health care fields.2
Healthcare workers (HCW) are key in this pandemic to ensure the continuous care of the overwhelming numbers of COVID-19 patients. Consequently, frontline HCW have a 3.4-fold higher risk than the general population to become infected.3 Not only are HCW highly exposed to SARS-CoV-2 due to their professional activity but they are also in close contact with vulnerable patients at high risk of severe COVID-19, which is why this group has been considered a priority for COVID-19 vaccination.4,5 Mathematical models support the benefits of frontline HCW vaccination in the reduction of deaths, hospital and ICU admissions from COVID-19.6 However, little is yet known about the effectiveness of these strategies.7
MethodsLa Paz University Hospital is a 1270-bed hospital with a catchment area of more than 500,000 individuals in the north of Madrid. During the pandemic, the hospital adapted its infrastructure in response to the high caseload, and by 12 February 2021, the hospital had attended 11,021 patients with COVID-19, one of the largest single-center cohorts in Europe.
Study design, period and participants: This is a prospective observational study carried out between 01/12/20 and 07/03/21. Our hospital has 7410 employees, which increased to 8329 in response to the high demand for care at the time of the epidemic.
Definitions: Confirmed case was defined as any healthcare worker with positive SARS-CoV-2 nucleic acid in a clinical specimen.
Epidemiological data collection: Data collection was daily carried out by the Occupational Health Department, in order to perform the clinical surveillance of employees. This Department was responsible also for the follow-up COVID-19 contact tracing and screenings, as well as HCW vaccination. Cumulative incidence of Madrid Region was obtained from Public Health weekly reports. Daily cases of total hospitalized COVID-19 patients were obtained from the Preventive Medicine Department records.
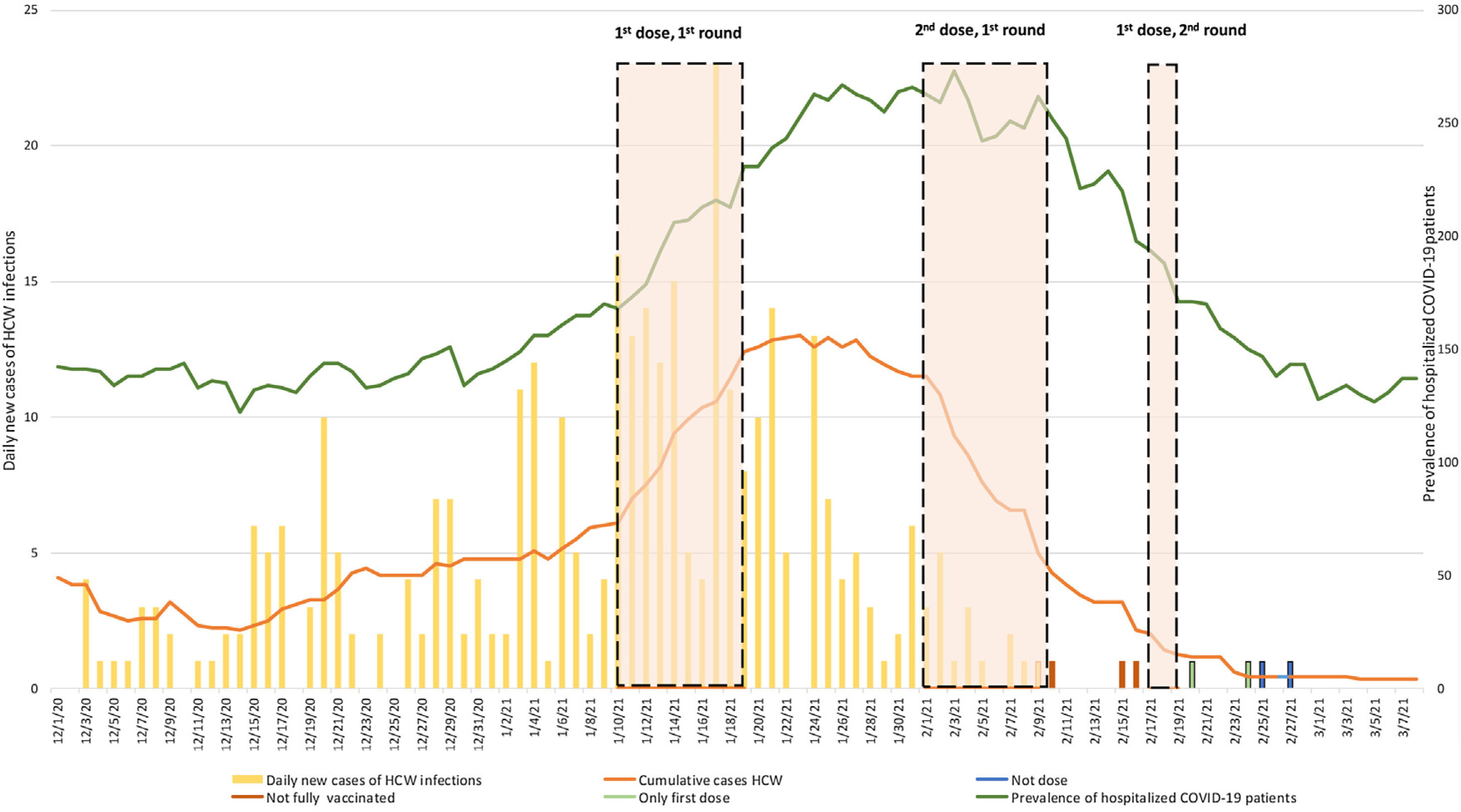
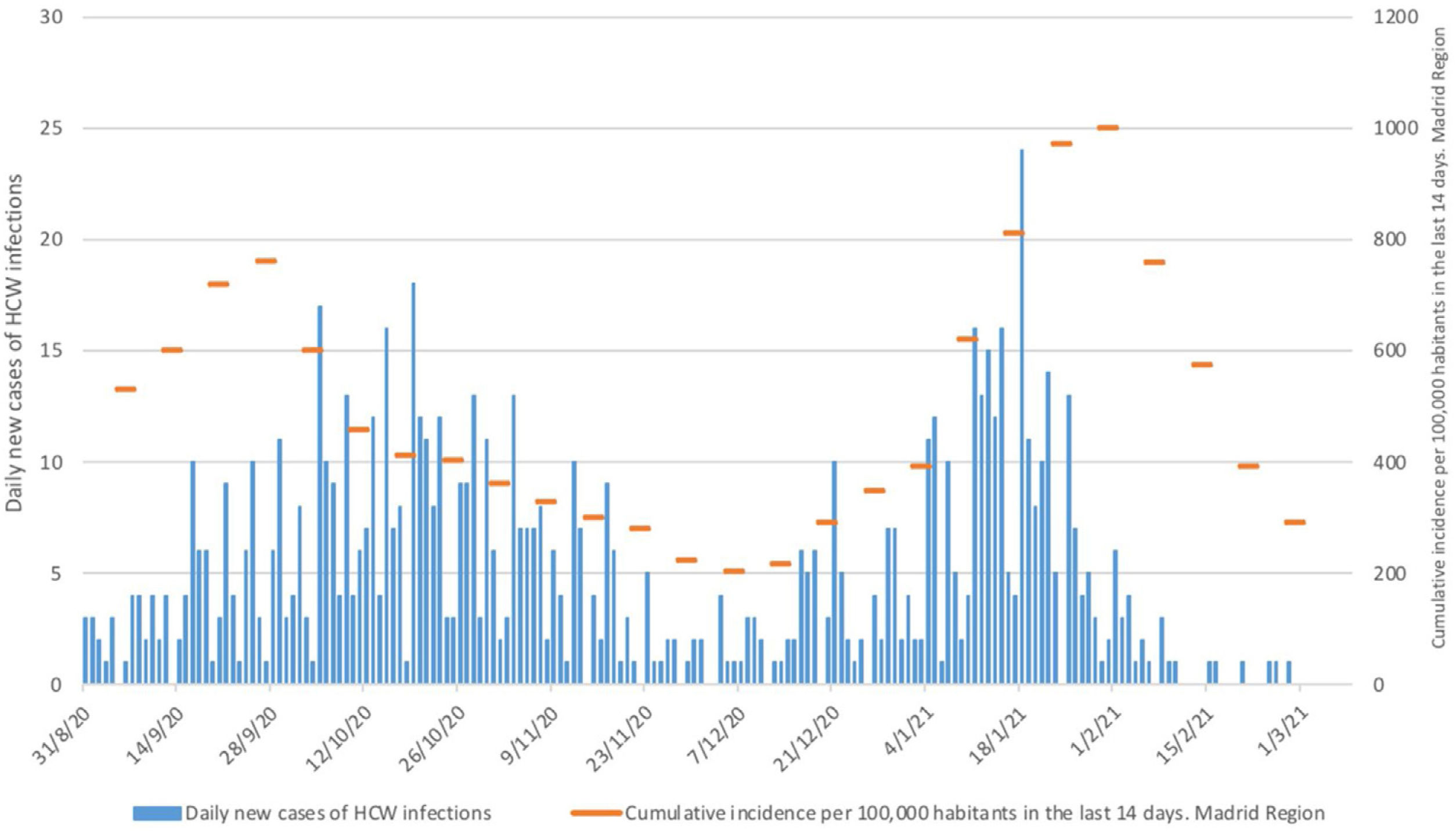
Results and discussionWe aimed to compare the incidence and prevalence of HCW infected pre-and post-vaccination with the BNT162b2 mRNA COVID-19 vaccine. The periods of vaccination were from 10/01/21 to 19/01/21 (first dose 7502 HCW) and from 01/02/21 to 09/02/21 (second dose 7423 HCW). For HCW who could not be vaccinated in this period of time (10,9%), a second round of vaccination started on a 17/02/21. Fig. 1 shows that the curves for the cumulative cases HCW and hospitalized COVID-19 patients paralleled during the so-called “second wave” of the pandemic in Madrid starting 31/08/20, that reached its peak on 31/1/21. Fig. 2 also shows that daily new cases of HCW infections in our hospital were in line with the 14 days cumulative incidence of COVID-19 cases per 100,000 habitants in the Madrid region in this time period.8
Since approximately two weeks after finishing the first round of vaccinations daily new cases of HCW infections (symptomatic and asymptomatic) decreased substantially and the cumulative cases of infected HCW and hospitalized COVID-19 patients started to diverge (Fig. 1). The curve of infected HCW decreased from a maximum of 157 on 23/10/20 to seven on 23/2/21. Daily incidence of new cases of infected HCW reached a maximum of 24 on 18/1/21 and decreased to zero after 16/2/21. This divergence between the prevalence curves is appreciable despite the fact that the 14 days cumulative incidence of COVID-19 cases is at present decreasing in Madrid. Of note, we have not seen any new case of HCW infection after seven days of the second dose of vaccination among the HCW (89% of the total) who have received the two doses of the BNT162b2 mRNA Covid-19 vaccine. Despite the presented data, the limitations of this type of observational study must be taken into account, including the potential for bias in the reporting of symptoms or positive tests.
To the best of our knowledge this is one of the first examples of the high effectiveness of a COVID vaccine in Spanish HCW. We can only hope to see, in the upcoming months, that this impressive benefit is also shared by the general population.
Conflict of interestsThe authors declare that they have no conflict of interest.
Occupational Health Department: Concepción Núñez López, Juan Manuel González, Natalia Arizaga Lobeto, Natalia Pérez Hidalgo, Maria Castiñeiras Ortega, Consuelo Rodrigo Garcia-Pando
Preventive Medicine Department: Verónica Pérez-Blanco, Claudia García-Vaz
Microbiology Department: Julio García Rodríguez, María Pilar Romero Gómez
Internal Medicine Department: Francisco Arnalich Fernández
Infectious Disease Unit: Rosa de Miguel Buckley, Marta Díaz-Menéndez, Jose R. Arribas.