Influenza is common in healthy children and adolescents and is associated with a high rate of hospitalization in this group, especially for those <5 years. Although the WHO has recommended vaccination in children under 5 years of age since 2012, it is really implemented in few countries today. The aim of this paper was to review the available evidence on the efficacy/effectiveness of influenza vaccination in healthy children <18 years of age through a non-systematic search of studies conducted between 2010 and 2020. Despite the high variability in results due to differences in design, vaccine type and season included in the 41 selected studies, statistically significant studies show efficacy values for the influenza vaccine of between 25.6% and 74.2%, and effectiveness from 26% to 78.8%. Although a systematic review would be necessary to corroborate the evidence, this review suggests that paediatric vaccination is generally an effective measure for preventing influenza in healthy children in line with international organisms’ recommendations.
La gripe tiene una elevada incidencia en niños y adolescentes sanos, y se asocia a una alta tasa de hospitalización, especialmente en los < 5 años. Desde 2012, la Organización Mundial de la Salud recomienda la vacunación en < 5 años, pero pocos países aplican hoy esta recomendación. El objetivo de este documento es revisar la evidencia disponible sobre la eficacia/efectividad de la vacunación antigripal en niños sanos < 18 años mediante una búsqueda no sistemática de estudios entre 2010-2020. A pesar de la gran variabilidad en los resultados debido a las diferencias de diseño, tipo de vacuna y temporada incluidos en los 41 estudios seleccionados, aquellos con significación estadística muestran valores de eficacia para la vacuna antigripal del 25,6% y al 74,2%, y de efectividad del 26% al 78,8%. Aunque sería necesaria una revisión sistemática para corroborar la evidencia, esta revisión sugiere que la vacunación pediátrica es globalmente una medida eficaz/efectiva para prevenir la gripe en niños sanos, en línea con las recomendaciones de organismos internacionales.
The influenza virus causes significant morbidity and mortality worldwide and has a considerable health and social impact on the paediatric population. Seasonal influenza infection is responsible for the hospitalization of almost 900,000 children under 5 years of age worldwide every year,1 the highest rates being observed in children under 2 years old.1,2 Most cases occur in children in developing countries, although in developed countries indirectly contributes to parental absenteeism.3 While the annual incidence of seasonal influenza infection is estimated at 5–10% in adults, rates among children are 20–30%.4 Furthermore, young children, especially schoolchildren, are the main vectors in the spread of the influenza virus among the population.5 In the family setting, this group appears to be the largest vehicle of transmission, given their close contact with adults and older people.5 Additionally, children shed larger amounts of virus and for a longer time (10–15 days) than adults and older people, making them “super-spreaders” of seasonal influenza epidemics.6
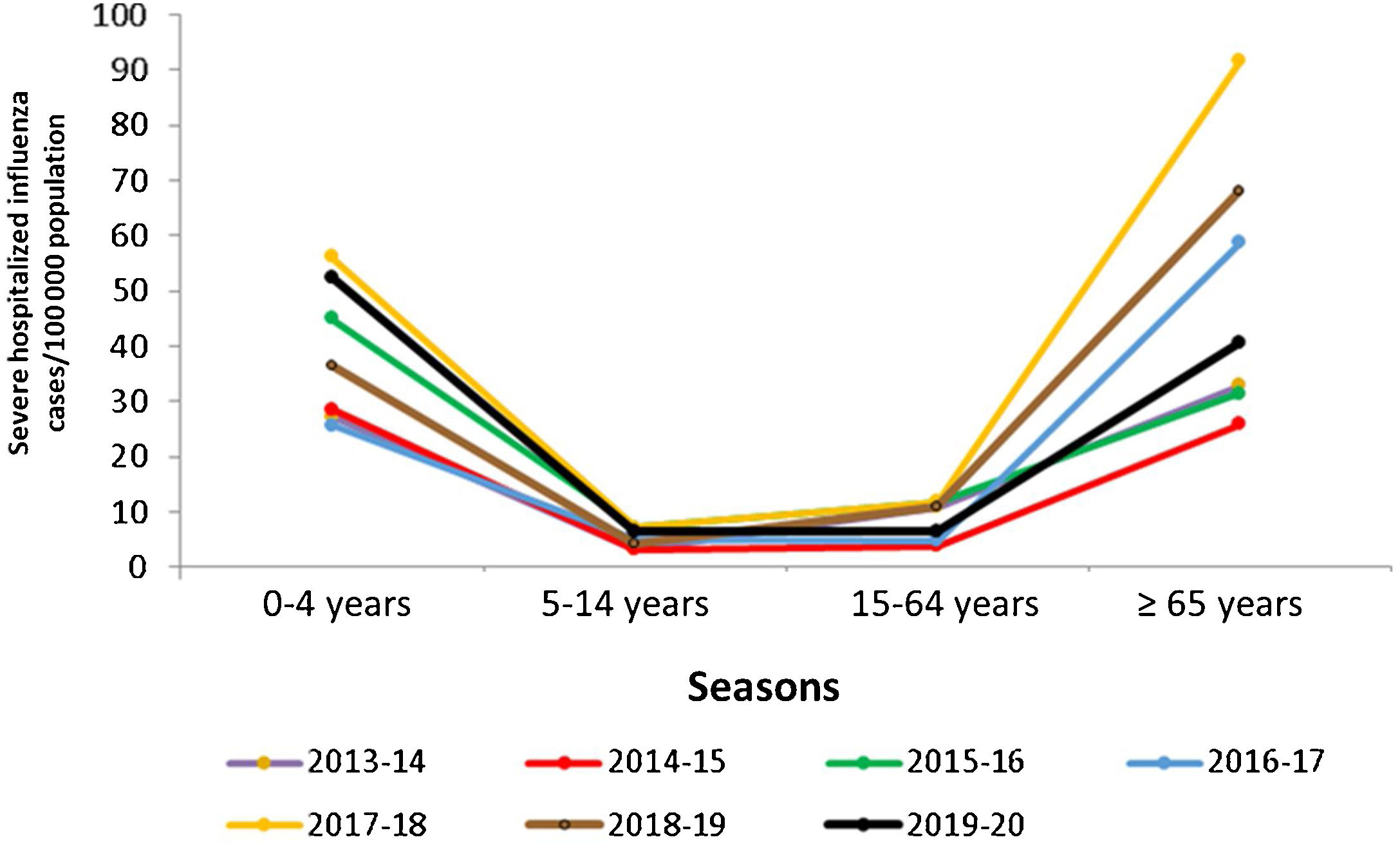
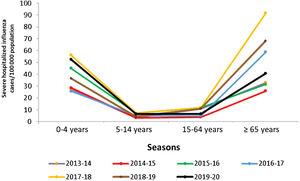
In Spain, influenza surveillance reports by the National Epidemiology Centre and the Carlos III Health Institute show the importance of the burden of influenza in normally healthy children (Table 1). The data show that in children under 15 years of age, those younger than 5 years have had the highest burden of disease in recent seasons, reaching a high level of incidence during the peak week of the epidemic wave (May 2020) in the influenza epidemic in the 2019–2020 season.7 This group presented the highest cumulative incidence rates of influenza (6244.7 cases/100,000 population), even higher than in individuals over 64 years of age (545.4 cases/100,000 population) as recorded in the previous two seasons.7–9 In some seasons, severe hospitalized cases of influenza in children equal or exceed hospitalization rates for adults over age 65, as demonstrated by the findings of the influenza surveillance network for 2015–2016 and 2019–2020 (Fig. 1).
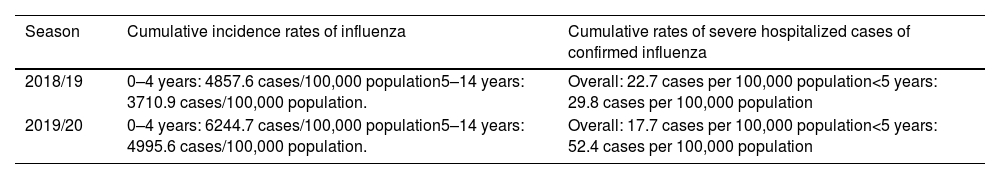
Impact of influenza in children (<15 years) in Spain in the 2018–2020 seasons.
| Season | Cumulative incidence rates of influenza | Cumulative rates of severe hospitalized cases of confirmed influenza |
|---|---|---|
| 2018/19 | 0–4 years: 4857.6 cases/100,000 population5–14 years: 3710.9 cases/100,000 population. | Overall: 22.7 cases per 100,000 population<5 years: 29.8 cases per 100,000 population |
| 2019/20 | 0–4 years: 6244.7 cases/100,000 population5–14 years: 4995.6 cases/100,000 population. | Overall: 17.7 cases per 100,000 population<5 years: 52.4 cases per 100,000 population |
Influenza vaccination is currently the most effective way of preventing both seasonal and pandemic influenza. Since 2012, the World Health Organization (WHO) has recommended the vaccination of children aged 6–59 months, pregnant women, older adults, people with chronic diseases, and healthcare providers.10 While some countries such as Finland, United Kingdom (UK) and Canada have included the paediatric population in their routine immunization schedules,11,12 others such as the United States (US) include the entire population from the age of 6 months under a universal vaccination recommendation. However, in most countries, including Spain, healthy children are not included as a target group in vaccination policies.13
The interterritorial council of the Spanish Ministry of Health has established the recommendation for influenza vaccination of children from the age of 6 months who have a chronic condition, history of prematurity or who may transmit influenza to those at high risk of complications.14 Recently, the Spanish Association of Paediatrics and the Vaccine Advisory Committee (CAV-AEP) has also recommended influenza vaccination of children over 6 months of age who are not included in risk groups.15
Given the burden of paediatric influenza and in the context of the actual pandemics caused by a respiratory disease, we considered the need of conducting a preliminary review of the most current scientific evidence on the efficacy and effectiveness of influenza vaccines in the paediatric population (between 6 months and 18 years) by analysing information from the main randomized clinical trials, real-life observational studies, and published systematic reviews/meta-analyses. The main objective was to obtain some insights into one of the important factors that need to be taken it into account when considering a hypothetical systematic vaccination in healthy children in Spain.
MethodsA review of the current literature was performed by searching for literature references in the electronic databases Medline/PubMed and Web of Science (WOS) and was restricted to articles published in English or Spanish between 2010 and 2020. The search strategy consisted of the following combination of terms for PubMed: “Influenza vaccines” [MeSH Terms] OR “Influenza vaccine*” [Title/Abstract] OR “Flu vaccine” [Title/Abstract]) AND (“Child” [MeSH Terms] OR “Child” [Title/Abstract] OR “Children” [Title/Abstract] OR “Childhood” [Title/Abstract]) AND (“Treatment Outcome” [MeSH Terms] OR “Efficacy” [Title/Abstract] OR “Effectiveness” [Title/Abstract]) AND (2010). The strategy for WOS was as follows: TS=(Influenza vaccines OR Influenza vaccine OR Flu vaccine) OR TI=(Influenza vaccines OR Influenza vaccine OR Flu vaccine) AND TS=(Child OR Children OR Childhood) OR TI=(Child OR Children OR Childhood) AND TS=(Treatment Outcome OR Efficacy OR Effectiveness) OR TI=(Treatment Outcome OR Efficacy OR Effectiveness).
We included original publications of clinical trials, observational studies, systematic reviews and meta-analyses that met the following inclusion criteria: they had to evaluate the efficacy/effectiveness of inactivated and live attenuated influenza vaccines approved for use in children against laboratory-confirmed influenza (defined by a positive PCR or viral culture result in nasopharyngeal samples) and include specific data in healthy children (6 months to 18 years) published during the ten reference years. The vaccine efficacy/effectiveness was considered statistically significant if the lower limit of the 95% CI was>0.
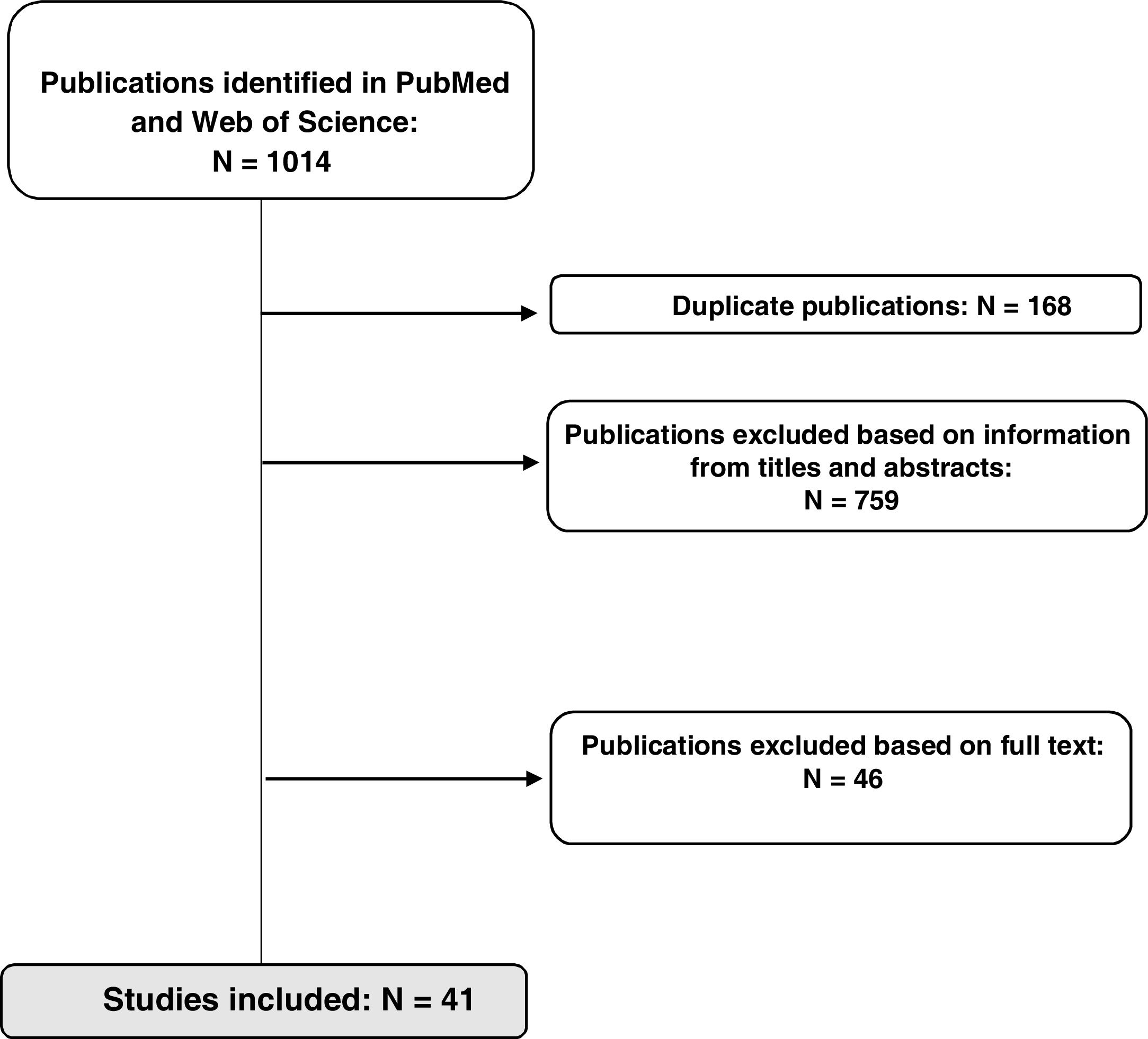
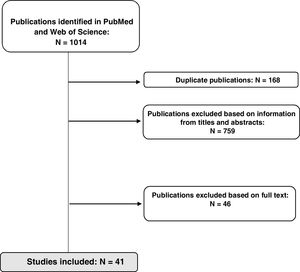
ResultsStudy selectionThe initial search identified a total of 1014 references in the two databases used; 168 duplicate references were removed. In a first review, 87 publications were selected after filtering according to information in the title and/or abstract, and another 46 were excluded after reading the full text because they did not meet the inclusion criteria. A total of 41 studies (11 clinical trials, 7 meta-analyses/systematic reviews, and 23 observational studies) were included in the final analysis, as shown in Fig. 2.
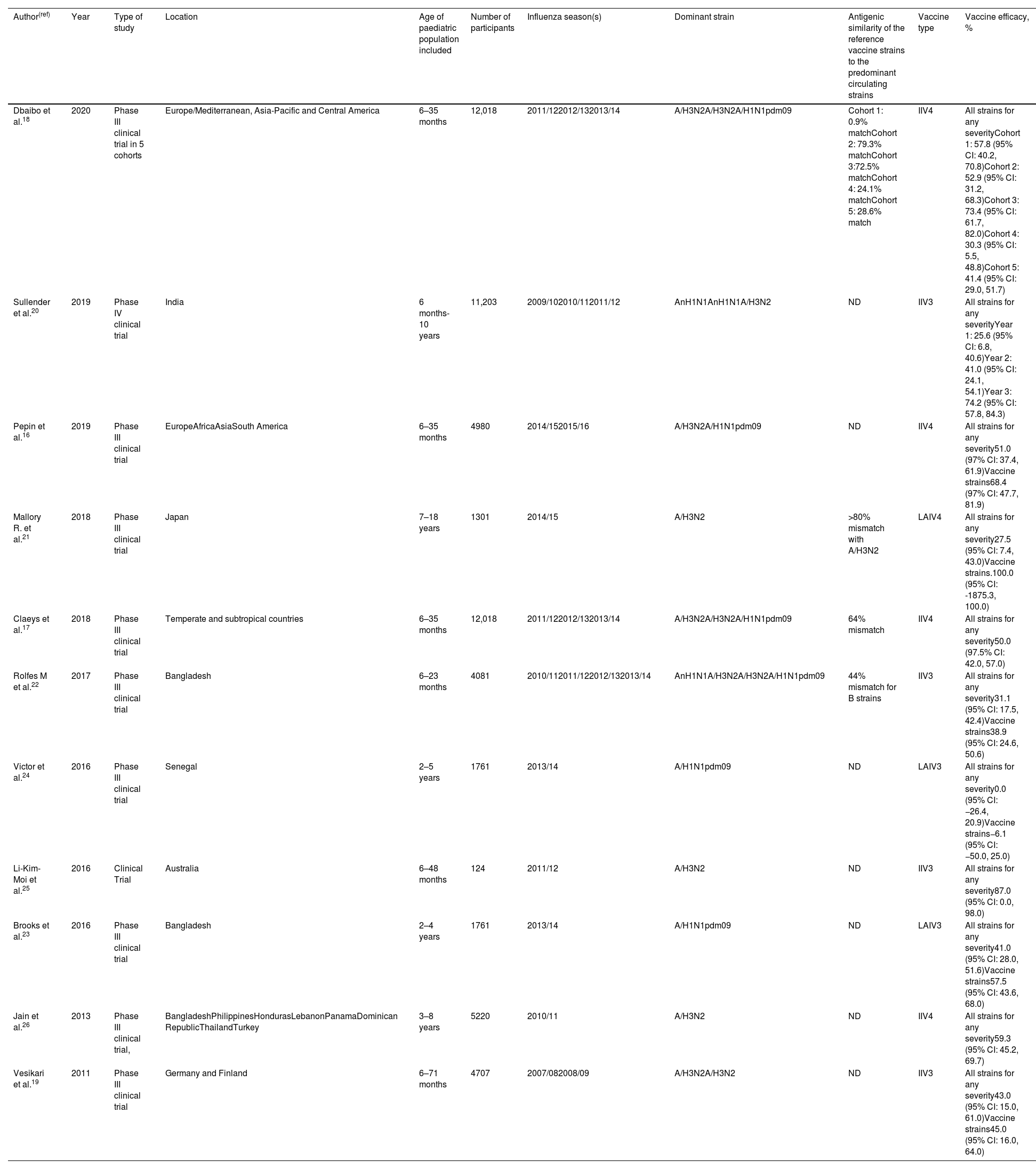
Clinical trialsEleven clinical trials evaluating the efficacy of influenza vaccination in children were included in the study (Table 2).16–26 Seven included children under the age of 2 in the analysis, a population in which there were few efficacy studies until recently. Overall, statistically significant results show that the efficacy of the IIV4 vaccine ranges from 30.3% (95% CI: 5.5, 48.8) to 73.4% (95% CI: 61.7, 82.0) for any virus. Similar values were observed for IIV3, with efficacy ranging from 25.6% (95% CI: 6.8, 40.6) to 74.2 (95% CI: 57.8, 84.3) according to the study. For live attenuated influenza vaccines (LAIV), effectiveness ranged from 27.5% (95% CI 7.4, 43.0) to 41.0% (95% CI: 28.0, 51.6). In children under 5 years of age, the overall mean efficacy of vaccines against all strains was between 25.6% (95% CI: 6.8, 40.6) and 87% (95% CI: 0, 98).
Efficacy of influenza vaccines against laboratory-confirmed influenza in children.
| Author(ref) | Year | Type of study | Location | Age of paediatric population included | Number of participants | Influenza season(s) | Dominant strain | Antigenic similarity of the reference vaccine strains to the predominant circulating strains | Vaccine type | Vaccine efficacy, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Dbaibo et al.18 | 2020 | Phase III clinical trial in 5 cohorts | Europe/Mediterranean, Asia-Pacific and Central America | 6–35 months | 12,018 | 2011/122012/132013/14 | A/H3N2A/H3N2A/H1N1pdm09 | Cohort 1: 0.9% matchCohort 2: 79.3% matchCohort 3:72.5% matchCohort 4: 24.1% matchCohort 5: 28.6% match | IIV4 | All strains for any severityCohort 1: 57.8 (95% CI: 40.2, 70.8)Cohort 2: 52.9 (95% CI: 31.2, 68.3)Cohort 3: 73.4 (95% CI: 61.7, 82.0)Cohort 4: 30.3 (95% CI: 5.5, 48.8)Cohort 5: 41.4 (95% CI: 29.0, 51.7) |
| Sullender et al.20 | 2019 | Phase IV clinical trial | India | 6 months-10 years | 11,203 | 2009/102010/112011/12 | AnH1N1AnH1N1A/H3N2 | ND | IIV3 | All strains for any severityYear 1: 25.6 (95% CI: 6.8, 40.6)Year 2: 41.0 (95% CI: 24.1, 54.1)Year 3: 74.2 (95% CI: 57.8, 84.3) |
| Pepin et al.16 | 2019 | Phase III clinical trial | EuropeAfricaAsiaSouth America | 6–35 months | 4980 | 2014/152015/16 | A/H3N2A/H1N1pdm09 | ND | IIV4 | All strains for any severity51.0 (97% CI: 37.4, 61.9)Vaccine strains68.4 (97% CI: 47.7, 81.9) |
| Mallory R. et al.21 | 2018 | Phase III clinical trial | Japan | 7–18 years | 1301 | 2014/15 | A/H3N2 | >80% mismatch with A/H3N2 | LAIV4 | All strains for any severity27.5 (95% CI: 7.4, 43.0)Vaccine strains.100.0 (95% CI: -1875.3, 100.0) |
| Claeys et al.17 | 2018 | Phase III clinical trial | Temperate and subtropical countries | 6–35 months | 12,018 | 2011/122012/132013/14 | A/H3N2A/H3N2A/H1N1pdm09 | 64% mismatch | IIV4 | All strains for any severity50.0 (97.5% CI: 42.0, 57.0) |
| Rolfes M et al.22 | 2017 | Phase III clinical trial | Bangladesh | 6–23 months | 4081 | 2010/112011/122012/132013/14 | AnH1N1A/H3N2A/H3N2A/H1N1pdm09 | 44% mismatch for B strains | IIV3 | All strains for any severity31.1 (95% CI: 17.5, 42.4)Vaccine strains38.9 (95% CI: 24.6, 50.6) |
| Victor et al.24 | 2016 | Phase III clinical trial | Senegal | 2–5 years | 1761 | 2013/14 | A/H1N1pdm09 | ND | LAIV3 | All strains for any severity0.0 (95% CI: −26.4, 20.9)Vaccine strains−6.1 (95% CI: −50.0, 25.0) |
| Li-Kim-Moi et al.25 | 2016 | Clinical Trial | Australia | 6–48 months | 124 | 2011/12 | A/H3N2 | ND | IIV3 | All strains for any severity87.0 (95% CI: 0.0, 98.0) |
| Brooks et al.23 | 2016 | Phase III clinical trial | Bangladesh | 2–4 years | 1761 | 2013/14 | A/H1N1pdm09 | ND | LAIV3 | All strains for any severity41.0 (95% CI: 28.0, 51.6)Vaccine strains57.5 (95% CI: 43.6, 68.0) |
| Jain et al.26 | 2013 | Phase III clinical trial, | BangladeshPhilippinesHondurasLebanonPanamaDominican RepublicThailandTurkey | 3–8 years | 5220 | 2010/11 | A/H3N2 | ND | IIV4 | All strains for any severity59.3 (95% CI: 45.2, 69.7) |
| Vesikari et al.19 | 2011 | Phase III clinical trial | Germany and Finland | 6–71 months | 4707 | 2007/082008/09 | A/H3N2A/H3N2 | ND | IIV3 | All strains for any severity43.0 (95% CI: 15.0, 61.0)Vaccine strains45.0 (95% CI: 16.0, 64.0) |
Vaccine efficacy was considered statistically significant if the lower limit of the 95% CI was>0. IIV3: Trivalent inactivated influenza vaccine; IIV4: Quadrivalent inactivated influenza vaccine; LAIV3: Trivalent live attenuated influenza vaccine; LAIV4: Quadrivalent live attenuated influenza vaccine; ND: Not determined.
The efficacy results of the clinical trial conducted by Victor et al.24 in Senegal were not statistically significant, showing lack of efficacy of an LAIV influenza vaccine administered in the 2013–2014 season (0.0%; 95% CI: −26.4, 20.9) although the study was done with a Russian LAIV vaccine not authorized by EMA neither by US CDC.24
Meta-analyses and systematic reviewsAmong the systematic reviews and meta-analyses that have been published in recent years, the Cochrane review was updated in 2018 (Table 3), which includes data from 20 clinical trials, 17 cohort studies, and 10 case-control studies in multiple influenza seasons.27 This update evaluated the efficacy and effectiveness of influenza vaccines in healthy children (between 6 months and 16 years), concluding that, in healthy children aged 2–16 years, influenza vaccines appear to reduce the number of laboratory-confirmed influenza cases compared to placebo. Specifically, LAIV reduced the risk of laboratory-confirmed influenza from a median of 18% to 4% (relative risk [RR]: 0.22; 95% CI: 0.11, 0.41) compared to placebo in children aged 3 to 16 years, with an efficacy of 78% (95% CI: 59, 89). Additionally, compared with placebo or not being vaccinated, IIVs reduced the risk of influenza from 30% to 11% (RR: 0.36; 95% CI: 0.28–0.48), with a moderate mean vaccine efficacy of 64% (95% CI: 52, 72).27
Summary of systematic reviews and meta-analyses evaluated on efficacy and effectiveness in healthy children.
| Author/year(ref) | Year | Included studies | Age of paediatric population included | Vaccines analyzed | Vaccine efficacy/effectiveness (VE)% (95% CI) |
|---|---|---|---|---|---|
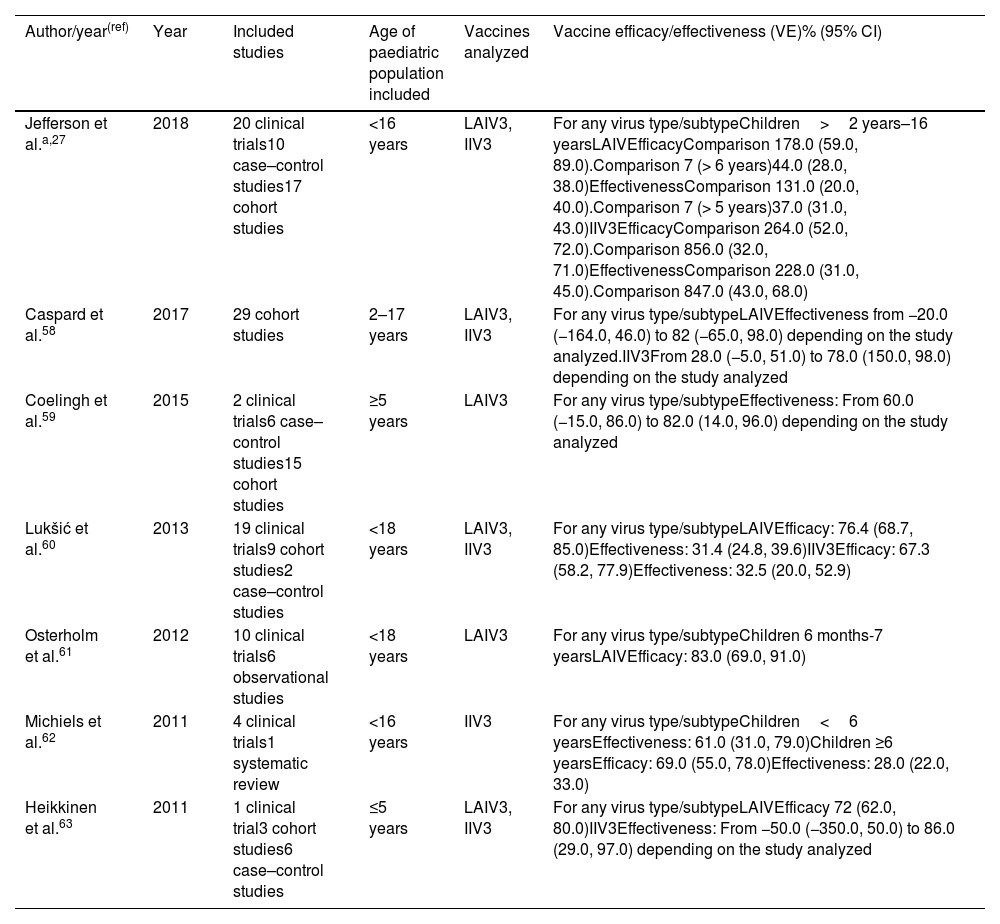
| Jefferson et al.a,27 | 2018 | 20 clinical trials10 case–control studies17 cohort studies | <16 years | LAIV3, IIV3 | For any virus type/subtypeChildren>2 years–16 yearsLAIVEfficacyComparison 178.0 (59.0, 89.0).Comparison 7 (> 6 years)44.0 (28.0, 38.0)EffectivenessComparison 131.0 (20.0, 40.0).Comparison 7 (> 5 years)37.0 (31.0, 43.0)IIV3EfficacyComparison 264.0 (52.0, 72.0).Comparison 856.0 (32.0, 71.0)EffectivenessComparison 228.0 (31.0, 45.0).Comparison 847.0 (43.0, 68.0) |
| Caspard et al.58 | 2017 | 29 cohort studies | 2–17 years | LAIV3, IIV3 | For any virus type/subtypeLAIVEffectiveness from −20.0 (−164.0, 46.0) to 82 (−65.0, 98.0) depending on the study analyzed.IIV3From 28.0 (−5.0, 51.0) to 78.0 (150.0, 98.0) depending on the study analyzed |
| Coelingh et al.59 | 2015 | 2 clinical trials6 case–control studies15 cohort studies | ≥5 years | LAIV3 | For any virus type/subtypeEffectiveness: From 60.0 (−15.0, 86.0) to 82.0 (14.0, 96.0) depending on the study analyzed |
| Lukšić et al.60 | 2013 | 19 clinical trials9 cohort studies2 case–control studies | <18 years | LAIV3, IIV3 | For any virus type/subtypeLAIVEfficacy: 76.4 (68.7, 85.0)Effectiveness: 31.4 (24.8, 39.6)IIV3Efficacy: 67.3 (58.2, 77.9)Effectiveness: 32.5 (20.0, 52.9) |
| Osterholm et al.61 | 2012 | 10 clinical trials6 observational studies | <18 years | LAIV3 | For any virus type/subtypeChildren 6 months-7 yearsLAIVEfficacy: 83.0 (69.0, 91.0) |
| Michiels et al.62 | 2011 | 4 clinical trials1 systematic review | <16 years | IIV3 | For any virus type/subtypeChildren<6 yearsEffectiveness: 61.0 (31.0, 79.0)Children ≥6 yearsEfficacy: 69.0 (55.0, 78.0)Effectiveness: 28.0 (22.0, 33.0) |
| Heikkinen et al.63 | 2011 | 1 clinical trial3 cohort studies6 case–control studies | ≤5 years | LAIV3, IIV3 | For any virus type/subtypeLAIVEfficacy 72 (62.0, 80.0)IIV3Effectiveness: From −50.0 (−350.0, 50.0) to 86.0 (29.0, 97.0) depending on the study analyzed |
Vaccine efficacy was considered statistically significant if the lower limit of the 95% CI was>0.
The results of the other systematic reviews and meta-analyses included in this study corroborate the evidence published by Cochrane, with mean statistically significant efficacy values of around 79% and effectiveness values of around 49% for LAIV. For inactivated vaccines, mean efficacy in these studies was 65%, while effectiveness ranged from 28% (95 CI%: 22, 33) to 86% (95% CI: 29, 97), depending on the study.
Observational studiesThe effectiveness of influenza vaccination in children has been analyzed in multiple studies, most of which were carried out by countries where universal vaccination is in place or where the vaccine in children is included in the standard vaccination calendar (Table 4). Of the 23 observational studies selected, more than half (14) included children under the age of 2. Overall, data show that the different types of influenza vaccines reduce the risk of laboratory-confirmed influenza by between 26% (95% CI: 15, 36) and 78.8% (95% CI: 66.9, 86.4). The variability observed in the reported values is due on the one hand, to the design and method of the study, and on the other, to the degree of similarity between the virus strains contained in the vaccine each year and the circulating strains of the study season.
Effectiveness of influenza vaccines against laboratory-confirmed influenza in the paediatric population.
| Authorref | Year | Season(s) | Country | Number of participants | Age of paediatric population included | Vaccines studied | Vaccine effectiveness% (95% CI) |
|---|---|---|---|---|---|---|---|
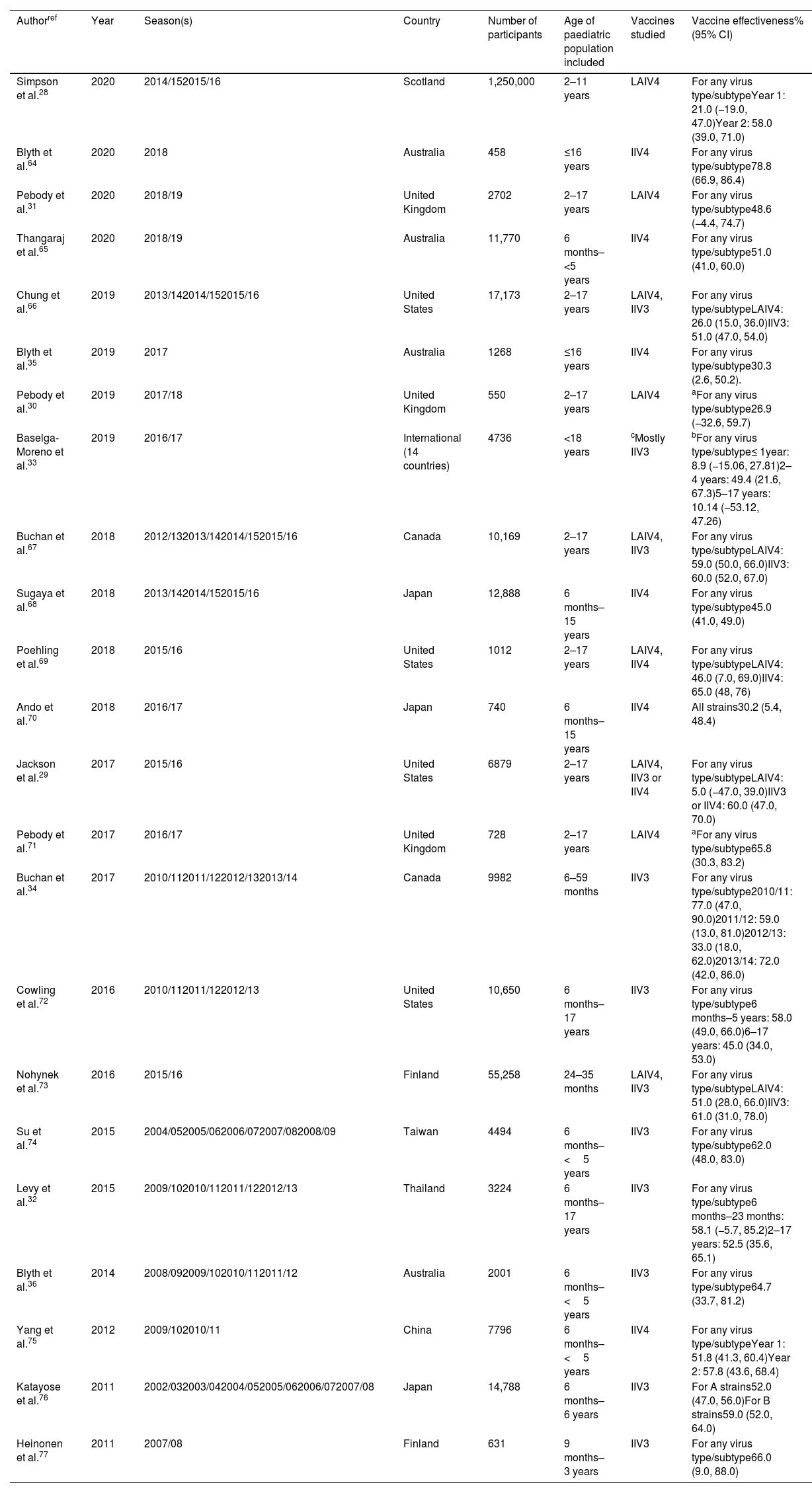
| Simpson et al.28 | 2020 | 2014/152015/16 | Scotland | 1,250,000 | 2–11 years | LAIV4 | For any virus type/subtypeYear 1: 21.0 (−19.0, 47.0)Year 2: 58.0 (39.0, 71.0) |
| Blyth et al.64 | 2020 | 2018 | Australia | 458 | ≤16 years | IIV4 | For any virus type/subtype78.8 (66.9, 86.4) |
| Pebody et al.31 | 2020 | 2018/19 | United Kingdom | 2702 | 2–17 years | LAIV4 | For any virus type/subtype48.6 (−4.4, 74.7) |
| Thangaraj et al.65 | 2020 | 2018/19 | Australia | 11,770 | 6 months–<5 years | IIV4 | For any virus type/subtype51.0 (41.0, 60.0) |
| Chung et al.66 | 2019 | 2013/142014/152015/16 | United States | 17,173 | 2–17 years | LAIV4, IIV3 | For any virus type/subtypeLAIV4: 26.0 (15.0, 36.0)IIV3: 51.0 (47.0, 54.0) |
| Blyth et al.35 | 2019 | 2017 | Australia | 1268 | ≤16 years | IIV4 | For any virus type/subtype30.3 (2.6, 50.2). |
| Pebody et al.30 | 2019 | 2017/18 | United Kingdom | 550 | 2–17 years | LAIV4 | aFor any virus type/subtype26.9 (−32.6, 59.7) |
| Baselga-Moreno et al.33 | 2019 | 2016/17 | International (14 countries) | 4736 | <18 years | cMostly IIV3 | bFor any virus type/subtype≤ 1year: 8.9 (−15.06, 27.81)2–4 years: 49.4 (21.6, 67.3)5–17 years: 10.14 (−53.12, 47.26) |
| Buchan et al.67 | 2018 | 2012/132013/142014/152015/16 | Canada | 10,169 | 2–17 years | LAIV4, IIV3 | For any virus type/subtypeLAIV4: 59.0 (50.0, 66.0)IIV3: 60.0 (52.0, 67.0) |
| Sugaya et al.68 | 2018 | 2013/142014/152015/16 | Japan | 12,888 | 6 months–15 years | IIV4 | For any virus type/subtype45.0 (41.0, 49.0) |
| Poehling et al.69 | 2018 | 2015/16 | United States | 1012 | 2–17 years | LAIV4, IIV4 | For any virus type/subtypeLAIV4: 46.0 (7.0, 69.0)IIV4: 65.0 (48, 76) |
| Ando et al.70 | 2018 | 2016/17 | Japan | 740 | 6 months–15 years | IIV4 | All strains30.2 (5.4, 48.4) |
| Jackson et al.29 | 2017 | 2015/16 | United States | 6879 | 2–17 years | LAIV4, IIV3 or IIV4 | For any virus type/subtypeLAIV4: 5.0 (−47.0, 39.0)IIV3 or IIV4: 60.0 (47.0, 70.0) |
| Pebody et al.71 | 2017 | 2016/17 | United Kingdom | 728 | 2–17 years | LAIV4 | aFor any virus type/subtype65.8 (30.3, 83.2) |
| Buchan et al.34 | 2017 | 2010/112011/122012/132013/14 | Canada | 9982 | 6–59 months | IIV3 | For any virus type/subtype2010/11: 77.0 (47.0, 90.0)2011/12: 59.0 (13.0, 81.0)2012/13: 33.0 (18.0, 62.0)2013/14: 72.0 (42.0, 86.0) |
| Cowling et al.72 | 2016 | 2010/112011/122012/13 | United States | 10,650 | 6 months–17 years | IIV3 | For any virus type/subtype6 months–5 years: 58.0 (49.0, 66.0)6–17 years: 45.0 (34.0, 53.0) |
| Nohynek et al.73 | 2016 | 2015/16 | Finland | 55,258 | 24–35 months | LAIV4, IIV3 | For any virus type/subtypeLAIV4: 51.0 (28.0, 66.0)IIV3: 61.0 (31.0, 78.0) |
| Su et al.74 | 2015 | 2004/052005/062006/072007/082008/09 | Taiwan | 4494 | 6 months–<5 years | IIV3 | For any virus type/subtype62.0 (48.0, 83.0) |
| Levy et al.32 | 2015 | 2009/102010/112011/122012/13 | Thailand | 3224 | 6 months–17 years | IIV3 | For any virus type/subtype6 months–23 months: 58.1 (−5.7, 85.2)2–17 years: 52.5 (35.6, 65.1) |
| Blyth et al.36 | 2014 | 2008/092009/102010/112011/12 | Australia | 2001 | 6 months–<5 years | IIV3 | For any virus type/subtype64.7 (33.7, 81.2) |
| Yang et al.75 | 2012 | 2009/102010/11 | China | 7796 | 6 months–<5 years | IIV4 | For any virus type/subtypeYear 1: 51.8 (41.3, 60.4)Year 2: 57.8 (43.6, 68.4) |
| Katayose et al.76 | 2011 | 2002/032003/042004/052005/062006/072007/08 | Japan | 14,788 | 6 months–6 years | IIV3 | For A strains52.0 (47.0, 56.0)For B strains59.0 (52.0, 64.0) |
| Heinonen et al.77 | 2011 | 2007/08 | Finland | 631 | 9 months–3 years | IIV3 | For any virus type/subtype66.0 (9.0, 88.0) |
Vaccine efficacy is considered statistically significant if the lower limit of the 95% CI is>0.
Adjusted for age, sex, smoking habits, social class, gastroenterologist visits in the last 3 months, obesity, pregnancy, influenza vaccination, time of sampling, season and site.
IIV3s were predominantly used among the 14 countries involved in the study.
IIV3: Trivalent inactivated influenza vaccine; IIV4: Quadrivalent inactivated influenza vaccine; aIIV3: Trivalent adjuvanted inactivated influenza vaccine; LAIV3: Trivalent live attenuated influenza vaccine; LAIV4: Quadrivalent live attenuated influenza vaccine.
The effectiveness results in six of the studies included in this analysis were not statistically significant, with effectiveness values ranging from 5% (95% CI: −47, 39) to 58.1% (95% CI: −5.7, 85.2), most of which (4 of 6) used LAIV vaccines.28–33
When effectiveness was analyzed according to the type of vaccine, LAIV vaccines were observed to have lower effectiveness values than inactivated vaccines (either trivalent or quadrivalent), ranging from an average of around 40% in the former to values greater than 50% in the latter. However, the use of LAIV vaccines is generally less extended than of IIV, thus there are fewer studies analysing the effect of LAIV vaccines with respect to IIV vaccines (8 vs 19 studies respectively among our selected observational studies).
DiscussionThis review is an approach to current published scientific evidence on influenza vaccination effectiveness in healthy children. The evaluation of the literature suggests that paediatric vaccination would be an effective measure to prevent influenza in this age group based on data showing statistically significant efficacy in clinical trials ranging from 25.6% (95% CI: 6.8, 40.6) to 74.2 (95% CI: 57.8, 84.3). Regarding the commonly subject to bias real-life observational studies, although some studies showed no effect, the statistically significant values range from 26% (95% CI: 15, 36) to 78.8% (95% CI: 66.9, 86.4) for any strain.
Many previous studies lacked sufficient evidence to determine the effect of the influenza vaccine in children under 2 years of age. However, several randomized clinical trials have been published in recent years including children from 6 months to 4 years of age,16–18,22,25 showing that the influenza vaccine appears to have efficacy in younger children as well, with results ranging between 25.6% (95% CI: 6.8, 40.6) and 87% (95% CI: 0, 98) depending on the trial. To this must be added the multiple cohort studies recently conducted in countries such as Canada or Australia (which have universal vaccination programmes that include children from the age of 6 months) with the aim of evaluating the effect of this measure on influenza prevention. The results also show that the effectiveness of the influenza vaccine appears to be significant in this age group.34–36 However, the data from the 6-month to 2-year subgroup should be independently analyzed to determine the specific effectiveness of this vaccine in this particular population group.
The results obtained after reviewing the observational studies are generally positive and appear to demonstrate the effectiveness of the influenza vaccine in healthy children. Nevertheless, they should be viewed with caution, because, given the nature of types of studies, there is a significant risk of bias. The great variability in the design and methodology of observational studies, together with the fact that the number of individuals were small and predominant influenza viruses varied by season can affect the results of this and other studies. In addition, studies have not considered the possible vaccine mismatch between vaccine and circulating virus, which may explain that data on vaccine effectiveness have wide confidence intervals. Consequently, care must be taken in drawing conclusions with observational studies. In fact, six of the observational studies analyzed do not show any statistically significant vaccine effectiveness,28–33 probably because most of them were conducted over a single season.
While data on vaccine efficacy and effectiveness appear robust in children under 5 years of age, evidence is scant in the 5–14-year-old age group, and even in young adults up to 18 years of age. Although the effects of influenza appear to be less severe in this age group, recent studies have shown that the average age of death from influenza in children with no previous conditions is around age 6–7 years.37 In addition, the important role of children as spreaders of the disease should be taken into account, so further studies should be conducted to determine the global effectiveness of the influenza vaccine in this age group.
Work has been underway for number of years in Europe to report the actual data on influenza vaccine effectiveness each season. The project, known as DRIVE (Development of Robust and Innovative Vaccine Effectiveness, part of the Horizon 2020 programme), has been providing high-quality information on the effectiveness of different influenza vaccine global manufacturers for three seasons.38 In the 2019/2020 season, 12 studies with a test-negative design (four in primary care and eight in hospitals) and one Finnish cohort study were analyzed. The values for vaccine effectiveness against laboratory-confirmed influenza in primary care in children aged 6 months to 17 years were between 64% (95% CI: 44, 80) and 66.3% (95% CI: 58.8, 72.4) for any vaccine (depending on the study design), which appears to be in line with the results obtained in our study. However, effectiveness against hospitalization was 33% (95% CI: -26%, 66%).38 These findings offer robust data on which decisions can be taken. In the 2020–2021 season, DRIVE aims to explore how the COVID-19 pandemic has affected the effectiveness of influenza vaccines.39
Childhood influenza vaccination should be an effective measure for reducing the overall burden of the disease and especially its serious consequences (hospitalization and complications) in children. In addition, childhood influenza vaccination impedes the transmission of influenza to vulnerable people and older people who respond less well to vaccination.40 Schoolchildren are effective and major spreaders of influenza2; almost half of influenza infections in children are asymptomatic or have very mild symptoms.41 In addition, children transmit the virus for a longer period and with higher viral loads, facilitating the indiscriminate spread of infection given the close contact of this population with other family members,2 who in turn can act as spreaders in their workplace. Evidence shows that influenza vaccination of schoolchildren not only protects vaccinated children, but also reduces exposure to the virus among people in the home environment and other members of their community. The first study to demonstrate this epidemiological effect was conducted by Arnold Monto in the US at the time of the pandemic outbreak of Hong Kong influenza. The researchers vaccinated schoolchildren in one community and not in another, but who both attended the same school. Vaccination achieved a 25–50% reduction in attack rates in the different age groups in the community where schoolchildren had been vaccinated with high coverage.42 A study in Japan showed that vaccinating schoolchildren was accompanied by a reduction in all-cause mortality and specifically deaths attributed to influenza, with the trend reversing when the policy was suspended in 1987. Vaccination of Japanese children prevented between 37,000 and 49,000 deaths per year, or approximately 1 death per 420 children vaccinated.43
Another Japanese study showed that vaccination of schoolchildren was associated with a 36% adjusted mortality reduction among older adults (≥ 65 years) due to influenza, thus preventing about 1000 deaths per year in this age group.44 Other studies have also demonstrated the ability of influenza vaccination in children to induce herd immunity. A US study revealed an inverse correlation between influenza vaccination coverage in children and the decline in influenza in the older people.45 In Canada, vaccination of children and adolescents in several Hutterite communities was 61% effective (95% CI: 8, 83) in preventing confirmed influenza in unvaccinated participants,46 and effectiveness was maintained in the following two seasons.47 In general, all studies focusing on this topic have shown that childhood vaccination with both inactivated and attenuated vaccines reduces influenza cases or associated clinical syndromes in adults.48 Furthermore, it is important to note that vaccination in children has been shown to be cost-effective.49,50 Studies carried out in European countries such as the Netherlands or Germany, or in American countries, such as Mexico or the US, have shown the financial viability of childhood vaccination.51–54
The current health crisis due to the COVID-19 pandemic and its clinical similarities to influenza in terms of paediatric presentation inevitably raise the question of the management and control of a future influenza epidemic or pandemic situation. In contrast to the data for SARS-CoV-2 infection available to date, children are and were also super-spreaders of the pandemic influenza A(H1N1)pdm09 strain in 2009.55 In this case, monovalent influenza vaccination proved to be highly effective in preventing influenza and its transmission.56 This high-effectiveness effect in a pandemic situation is logical because the causative agent is a new virus against which even heterotypic protection is nil in paediatric cohorts.
Despite the generally positive scientific evidence found in this review, many other factors might be considered in the evaluation of the inclusion of a vaccine in the vaccination calendar. Vaccine efficacy/effectiveness is one of the most important aspects to be analyzed. But there are other considerations that need to be taken it into account such as the burden of the disease, vaccine safety, ethical aspects and economic implications.57 Thus, it is a complex process that involves many different agents and requires some time.
There are some limitations in this review that should be stated. Although we performed a detailed review of the actual evidence, this is not a systematic review. We have only used two databases (Medline/PubMed and Web of Science) for the searching of the studies; thus, we could not rule out the possibility of having excluded some articles. Also, the analysis of the selected articles has not been performed in a systematic way, therefore limiting our ability to conduct a thorough evaluation of all the results. On the other hand, the heterogenicity in the design of the included observational studies difficult the direct comparison of the results. The scarce number of studies analysing children aged 6 months to 2 years is also a weakness that needs to be considered.
Despite these limitations, this review supposes a good starting point to initiate the debate regarding the need to vaccinate healthy children in Spain, now that the Spanish Association of Paediatricians has recently advised the vaccination of all children over 6 months of age. A timely meta-analysis/systematic review of this topic would be needed to provide stronger evidence and would help resolve possible discrepancies in the literature. However, evidence found in this review, together with the WHO and European Centre for Disease Control (ECDC) recommendations, as well as experiences in other European countries and the need to be prepared for a future influenza pandemic, would support the inclusion of influenza vaccination in healthy children, specifically those under 5 years of age, in the routine Spanish vaccination schedule.
FundingSanofi has sponsored the development of this work by funding medical writing services and facilitating expert panel meetings. The authors have developed and agreed on the content, regardless of the source of funding.
Conflicts of interestROL has received fees for conferences and scientific advice from AstraZeneca, GSK, MSD, Sanofi and Seqirus. JAA has participated in teaching activities funded by AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi Pasteur, as clinical trial researcher at GlaxoSmithKline, Novartis and Sanofi Pasteur and as consultant on the Advisory Board of AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi Pasteur. MGS has collaborated in educational activities supported by GlaxoSmithKline, MSD, Pfizer and Sanofi Pasteur, as a researcher in clinical trials for GlaxoSmithKline, Pfizer, MSD and Sanofi Pasteur and as a consultant on the Advisory Board of AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi Pasteur. AVA has collaborated in teaching activities supported by GlaxoSmithKline and Pfizer and as a researcher in clinical trials for Pfizer and Sanofi Pasteur. AOS has attended to several conferences whose registration, travel and accommodation costs have been covered by pharmaceutical companies. AOS and his institution received research grants to develop observational studies and clinical trials from SP, GSK and MSD and some of them were related to the Influenza vaccines. AOS also acted as advisor for GSK and MSD for other vaccines”.
The authors would like to thank Dr. Vanessa Marfil, PhD (Medical Statistics Consulting, Valencia, Spain) for her assistance in the drafting and editing of this manuscript.