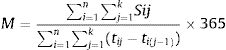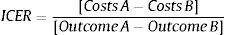Calculate the efficiency of the EmERGE Pathway of Care for medically stable people living with HIV at the Hospital Clínic-IDIBAPS, Barcelona, Spain.
Methods546 study participants were followed between 1st July 2016 and 30th October 2019 across three HIV outpatient clinics, but the virtual clinic was closed during the second year. Unit costs were calculated, linked to mean use outpatient services per patient year, one-year before and after the implementation of EmERGE. Costs were combined with primary and secondary outcomes.
ResultsAnnual costs across HIV-outpatient services increased by 8%: €1073 (95%CI €999–€1157) to €1158 (95%CI €1084–€1238). Annual cost of ARVs was €7,557; total annual costs increased by 1% from €8430 (95%CI €8356–8514) to €8515 (95%CI €8441–8595).
Annual cost for 433 participants managed in face-to-face (F2F) clinics decreased by 5% from €958 (95%CI 905–1018) to €904 (95%CI 863–945); participants transferred from virtual to F2F outpatient clinics (V2F) increased their annual cost by a factor of 2.2, from €115 (95%CI 94–139) to €251 (95%CI 219–290). No substantive changes were observed in primary and secondary outcomes.
ConclusionEmERGE Pathway is an efficient and acceptable intervention. Increases in costs were caused by internal structural changes. The cost reduction observed in F2F clinics were off-set by the transfer of participants from the virtual to the F2F clinics due to the closure of the virtual clinic during the second year of the Study. Greater efficiencies are likely to be achieved by extending the use of the Pathway to other PLHIV.
Calcular la eficiencia de la vía asistencial EmERGE para personas clínicamente estables que viven con VIH en el Hospital Clínic-IDIBAPS, en Barcelona, España.
MétodosSe realizó un seguimiento a 546 participantes del estudio, entre el 1 de julio de 2016 y el 30 de octubre de 2019, en tres clínicas ambulatorias de VIH, pero la clínica virtual se cerró durante el segundo año. Se calcularon los costes unitarios, vinculados al uso medio de los servicios ambulatorios por paciente al año, un año antes y después de la implementación de EmERGE. Los costes se combinaron con criterios de valoración principales y secundarios.
ResultadosLos costes anuales en los servicios ambulatorios para el VIH aumentaron un 8%: 1.073 € (IC 95%: 999-1.157 €) a 1.158 € (IC 95%: 1.084-1.238 €). El coste anual de los fármacos antirretrovirales (ARV) fue de 7.557 €; los costes anuales totales aumentaron en un 1%, de 8.430 € (IC 95%: 8.356-8.514 €) a 8.515 € (IC 95%: 8.441-8.595 €).
El coste anual para 433 participantes que recibieron tratamiento en clínicas presenciales (face to face, F2F) disminuyó en un 5%, de 958 € (IC 95%: 905-1.018 €) a 904 € (IC 95%: 863-945 €); los participantes transferidos de clínicas ambulatorias virtuales (V2F) a F2F aumentaron su coste anual en un factor de 2,2, de 115 € (IC 95%: 94-139 €) a 251 € (IC 95%: 219-290 €). No se observaron cambios sustanciales en los criterios de valoración principales y secundarios.
ConclusiónLa vía EmERGE es un tratamiento eficaz y aceptable. Los aumentos de los costes fueron el resultado de cambios estructurales internos. La reducción de costes observada en las clínicas F2F se compensó con la transferencia de participantes de las clínicas virtuales a las F2F debido al cierre de la clínica virtual durante el segundo año del estudio. Es probable que se logre una mayor eficiencia si se amplía el uso de la vía a otras personas que viven con VIH (PVVIH).
The 2020 Spanish Covid19 epidemic reiterated the need for people living with chronic diseases to remain in touch with care-givers, especially during emergencies.1 The global HIV community increasingly wants to track, monitor and evaluate the use, cost, outcome and impact of national health services for people living with HIV (PLHIV).2 Mobile Health (mHealth), employs wireless technology to deliver health information3,4 and plays an increasingly important role in linking care and integrating health services.5 mHealth has become an important tool to respond to the Covid-19 Pandemic.6,7
Increased global access to anti-retroviral drugs (ARVs),8 resulted in increased life-expectancy of PLHIV, similar to that of people not living with HIV.9 This will increase the number of PLHIV, including those aged 50 years or older.10 Non-HIV cancers, cardiovascular disease and other non-communicable diseases (NCDs) are now the commonest cause of death for PLHIV in high income countries,11 while the incidence and prevalence of NCDs are increasing among PLHIV in low- and middle-income countries.12 PLHIV will increasingly need to use HIV and non-HIV health and social services and integrated health information systems can assist service integration and make them more cost-effective.5
The Evaluating mHealth technology in HIV to improve Empowerment and healthcare utilisation: Research and innovation to Generate Evidence for personalised care (EmERGE) Project developed and implemented a new mHealth Pathway in five HIV centres in five European countries: Belgium, Croatia, England, Portugal and Spain. The Pathway includes a mobile health application (App), which enables PLHIV access to communicate with care-givers and personal health information.13 Recruitment criteria are described in Table 1.
Inclusion and exclusion criteria for EmERGE participants [14].*.
| Inclusion criteria: Patients who meet all of the following criteria were eligible for this study: | • Documented HIV infection |
| • Aged at least 18 years old | |
| • Able to give informed consent | |
| • In possession of a smartphone, tablet, or similar technology supporting the mHealth platform | |
| • Clinically stable on anti-retroviral therapy (ART). This was defined as receiving ART for at least 1 year and unchanged regimen for at least 3 months, 2 consecutive undetectable viral load measures (<50copies/ml), no current pregnancy and without any new WHO clinical stage 2, 3 or 4 events within the previous 12 months* | |
| Exclusion criteria: Patients who met one or more of the following criteria were excluded from the study | • Aged less than 18 years |
| • Pregnant | |
| • Participating in a clinical trial or receiving an investigational medication | |
| • Unable to comprehend the patient information sheet | |
| • Unable to comprehend the instructions for using the mHealth platform | |
| • Considered for any other reason by their regular physician to be unsuitable for study participation | |
A recent review identified nine communication functions for a mHealth App,4 but missed two important criteria: firstly, the confidentiality and security of the data need to be protected. Secondly, the technology needs to be affordable and efficient (Table 2; 15).
| 1. Patient-provider and peer communication* |
| 2. Medication and appointment reminders* |
| 3. A medication checklist, pill identification function and list of current and discontinued medicines* |
| 4. Laboratory reports (CD4 count, viral load, sexually transmitted infections, glucose and complete blood count)* |
| 5. Pharmacy information* |
| 6. Nutrition and fitness trackers |
| 7. Resources, links to social services, substance abuse support, video testimonials, case-management* |
| 8. Settings (profile picture, password and alerts)* |
| 9. A search function* |
| 10. Protecting the confidentiality and security of personal information at rest and in-transit** |
| 11. Affordability and efficiency of the technology** |
Most mHealth studies have not included the cost of developing and implementing these mHealth Apps, nor their efficiency. The need for efficiency studies was recognised long ago,16,17 but few efficiency studies have been performed since.18
This before-and-after study calculated the use, cost and cost-effectiveness of HIV services for EMERGE participants one-year pre- and one-year post-implementation of the EmERGE Pathway at the Hospital Clinic-IDIBAPS (HC- IDIBAPS), University of Barcelona, Barcelona, Spain. The specific objectives were: (1) calculate the use of HIV outpatient services at HC- IDIBAPS pre- and post-EmERGE; (2) calculate the unit costs of departments supporting HIV outpatient services; (3) calculate the annual costs of HIV outpatient services pre- and post-EmERGE; (4) calculate the efficiency of implementation of the EmERGE Pathway at HC-IDIBAPS.
MethodsContext546 participants were followed up between 1st July 2016 and 30th October 2019 at the Instituto Clinic de Medicina y Dermatología (ICMiD). The ICMiD is a research centre of the HC-IDIBAPS, and provides the infectious diseases service for this Hospital.
At the start of the study, there were three separate outpatient clinics where PLHIV were followed up: General Outpatient Clinic (Dispensario), the HIV Daycare Hospital and the ICMiD Daycare. While the former two conducted face-to-face appointments the latter clinic offered PLHIV virtual appointments. This video-consultation appointment system – Hospital Virtual – had been in operation since 2005.19 During the second year of the study the virtual clinic was closed for internal technical reasons. The videoconferencing and sound system in use was old and inadequate. As this could not be upgraded in a timely manner to continue services, this service was therefore closed during the second year of the study. As a consequence, those participants that were recruited to attend the virtual clinic during the first year of the study, were transferred to the two face-to-face clinics during the second year. The virtual clinic was closed in October 2019.
At the time of recruitment EmERGE participants signed an informed consent form, and this visit was the base-line for the analyses. Participants were followed up one year retrospectively pre-EmERGE and prospectively post-EmERGE implementation. All investigative procedures and minor surgical procedures were performed in the ICMID Daycare Office or HIV Daycare Hospital.
Tests results were transmitted electronically, reviewed and stored in electronic medical records. ARVs were provided free and most participants collected them from the Hospital. HIV outpatient services were supported by the following departments: laboratories, pharmacy, surgical and radiology. The HC-IDIBAPS developed its own method to cover overheads. It provides departments with a fixed additional percentage of their budget to cover the following overheads: cleaning & waste, laundry & clothes, materials, messaging & communication, security, information technology services, legal, administrative, human resources, financial, and managerial services. None of EmERGE participants had inpatient stays during the study period, hence the inpatient ward was not costed. As per EmERGE protocol, one visit per year was an electronic ‘visit’ when the physician reviewed the results of blood tests and sent their recommendation for future treatment via the EmERGE App; the other annual visit, part of routine management, was a face-to-face visit, or virtual visit for the ICMiD Daycare Outpatient participants during their first year of the study.
Data collectedInformation on the use, cost and outcome of HIV services by EmERGE participants pre- and post-EmERGE were required to estimate efficiency of the Pathway. This required process information on use of services, the unit costs of the services used, average annual cost of services pre- and post-EmERGE and changes in primary or secondary outcome measures.
Costing health facilities: general principlesThe “top-down” or “bottom-up or ingredient-based” methods are the two main costing methods.20,21 The more detailed “bottom-up” method defines the type and quantity of input or ingredients used to produce the service output. The ‘top-down’ method is easier to perform and determines the total cost of providing services on accounts of past expenditure. This expenditure is then divided by the number of ‘products’ provided during the period. Where possible, the preferred ingredient-based approach was used, but this was dependent on the availability of detailed data.
Process dataThe use of services by EmERGE participants in the year pre- and post-EmERGE provided the individual process data, which were obtained from the electronic medical records. They predominantly used outpatient services and therefore the focus of the costing exercise was on the three HIV outpatient services at HC-IDIBAPS.
Unit costs dataA micro-costing study was performed between October and November 2018. The focus was on outpatients’ services used by EmERGE participants for the 2018 financial year. The costing study was based on the UNAIDS Costing Manual20 and UNAIDS Costing Workbook.21 To estimate the unit costs, workload and financial data were collected from departments supporting EmERGE outpatient services. The individual process data of EmERGE participants identified which services were used and these were costed. The financial data included staff, consumables, overhead, procedures and equipment costs (SCOPE).22 Unit costs of outpatient services were combined with process data to calculate average annual costs of outpatient services for participants pre- and post-EmERGE.23
Statistical methodsAll participants with a baseline visit, contributed to the data and analyses presented. Summary statistics are presented with point estimates and indication of variability and missing data. Linear mixed models were used to calculate difference in three-monthly averages in CD4 counts and viral load measurements (DAVG; 24). Time weighted changes of CD4 count and viral load were analysed over a two-year period and measured by time point changes during one year before recruitment and one year after EmERGE recruitment.25 MIXED procedure in SAS was used by fitting routine values of CD4 counts and viral loads results as dependent variables.25 Independent variables included the fixed effects of study visit time points and time in months. A covariance matrix was used to model the within patient errors. Estimates of effects are based on MIXED models and assume any missing data were missing at random. Trends over time are presented as point estimates derived from the models. Viral load data were transformed logarithmically to stabilise their variance.
The mean number of services used per patient year (MPPY) was calculated using methods employed previously,26–28 based on the following formula:
Equation 1.1 Mean Per Patient Year (MPPY) Calculation Formula
where n=total number of individuals; k=day of censoring; Sij=use of service by individual i on jth day; tij=number of days of follow up for individual i; M=mean of services S per patient-year.The denominator comprised the total duration of follow up for all patients during a calendar year, one year from baseline and one-year prior to study entry. The data were left censored at the one-year pre-baseline visit. Post-EmERGE data were right censored at either one year since baseline visit if patients were still under follow-up at one year, or if they had died during follow up then their date of death, or if they were lost to follow up, which ever came first. Numerators were calculated by summing the use of outpatient services (Eq. 1.1). Exact Poisson 95% confidence intervals (CIs) were estimated for MPPY based on the distribution of the observed number of outpatient visits divided by the total duration of follow up for all patients during a calendar year. All statistical analyses were performed using SAS version 9.4 software.25
Average annual costs per patient-year (PPY) of EmERGE outpatient service provision were produced by multiplying the MPPY by their respective unit costs. The total average annual costs for providing services was obtained by adding the annual costs for outpatient visits, tests, procedures and drugs at the HC- IDIBAPS.22,23 Costings were performed from a societal perspective.
The original focus of the analysis was the use and costs for different HIV outpatient clinics, but as the Virtual Clinic was closed during the second year, an additional analysis was performed to compare the use and cost of participants followed up only in face-to-face clinics (F2F) with those participants that were transferred from the virtual to the face-to-face clinics during the second year of the study (V2F).
Primary and secondary outcome measuresChanges in CD4 count and viral load measurements were primary outcome measures pre- and post-EmERGE. The secondary outcome measures included changes in patient activation, measured by PAM13,29 and PROQOL-HIV quality of life measures,30 from month 0 (baseline) to month 12 post-EmERGE.
Cost-effectiveness analysesIncremental cost-effectiveness ratios (ICERs) were calculated based on the average annual costs, primary and secondary outcome measures before and after the implementation of EmERGE using the following formula31:
As the primary and secondary outcome measures did not change substantially between periods, the study ended up being a cost-minimization study comparing annual costs between periods.
Out of pocket expenditure for EmERGE participantsInformation was also collected from EmERGE participants’ socio-economic background, time off work for clinic appointments, return travel time and costs for clinic appointments.
Results546 participants were followed up between July 2016 and October 2019, of whom 21% (n=113) were seen in the Virtual Clinic pre-EmERGE; 92% of all participants were men and mean age at study entry was 43.3 years (95%CI 42.0–44.6). Median age was 43.0 years (IQR 36.9–49.4) and age at time of baseline ranged from 23.7 to 70.1 years.
Of those participants who self-identified their ethnicity, 77% were Caucasian, 20% Hispanic, 1% Black and 3% ‘other’. 76% described themselves as ‘men who have sex with men’, 14% heterosexuals and two percent as bisexual.
Of participants with known employment status, 84% had fulltime employment of a median 40hours working-week (IQR 34–40) and a median monthly income of €1450 (IQR €1050–€2050). Five percent received additional social services benefits; 52% received income support, 49% housing benefits and 24% received pension credits. Median monthly additional income was €465 (IQR €400–€738).
Median number of sick days three month prior to enrolment was 0 days (IQR 0–0). Around half of the participants took a day off for professional consultations, blood tests or pick up medication. The median return travel time to the HC-IDIBAPS was 1.5h (IQR: 0.5–2.) and median cost was €3 (IQR: €0–8).
Estimated unit costsThe unit costs estimated through the micro-costing exercise, are displayed in Table 322: the unit cost for a virtual clinic visit was less than that for face-to-face visits.
Unit costs of HIV outpatient services for HC-IDIBAPS EmERGE participants.
| HC- IDIBAPS | Cost/unit cost |
|---|---|
| Unit cost per EmERGE Participant Dispensario clinic Outpatient visit | €173 |
| Unit cost per EmERGE Participant ICMiD Office Virtual Outpatient visit | €51 |
| Unit cost per EmERGE Participant Day-care Hospital Outpatient visit | €118 |
| Unit cost per EmERGE Participant Hospital Outpatient visits across all Clinics | €114 |
| Unit cost per EmERGE Participant ICMiD Office Anal Canal Day ward Procedure | €398 |
| Unit cost per EmERGE Participant Day-care Hospital Fibroscan Day ward Procedure | €64 |
| Unit cost per EmERGE Participant Day-care Hospital Dexascan Day ward Procedure | €64 |
| Unit cost per EmERGE Participant Pharmacy patient excluding ARVs costs | €45 |
| Annual cost of ARVs for EmERGE Participant | €7357 |
| Unit cost per EmERGE Participant Biochemistry Laboratory test | €0.7 |
| Unit cost per EmERGE Participant Haematology Laboratory test | €2.0 |
| Unit cost per EmERGE Participant Microbiology Laboratory test | €13 |
| Unit cost per EmERGE Participant Immunology Laboratory test | €20 |
| Unit cost per EmERGE Participant Radiology investigation | €39 |
Across all clinics, outpatient visits increased between periods from 5.2 MPPY (95%CI: 5.0–5.4) to 5.6 MPPY (95%CI: 5.4–5.8) an increase of 8% (Table 4).
Annual mean use and cost PPY HIV outpatient visits, tests and procedures, pre- and post-EmERGE implementation at the HC-IDIBAPS.
| N=546 | Pre-EmERGE | Post-EmERGE | ||
|---|---|---|---|---|
| Mean/average | 95% CI interval | Mean/average | 95% CI interval | |
| Mean Dispensario Outpatient visits PPY and average costs PPY | 2.1 | 2.0–2.3 | 2.3 | 2.2–2.5 |
| € 368 | € 346–€ 391 | € 401 | € 379–€ 424 | |
| Mean ICMiD Office virtual visit and average costs PPY | 0.6 | 0.5–0.6 | 0.05 | 0.04–0.08 |
| € 28 | € 24–€ 32 | € 3 | € 2–€ 4 | |
| Mean Daycare Outpatient visits and average costs PPY | 2.6 | 2.4–2.7 | 3.2 | 3.0–3.3 |
| € 300 | € 284–€ 318 | € 374 | € 357–€ 392 | |
| Outpatient visit MPPY | 5.2 | 5.0–5.4 | 5.6 | 5.4–5.8 |
| TOTAL average cost visits PPY | € 696 | € 654–€ 741 | € 778 | €738–€ 820 |
| Mean biochemistry tests and average costs PPY | 51.0 | 50.4–51.7 | 36.7 | 36.1–37.2 |
| € 36 | € 35–€ 36 | € 26 | € 25–€ 26 | |
| Mean haematology tests and average costs PPY | 11.3 | 11.0–11.6 | 8.4 | 8.1–8.6 |
| € 23 | € 22–€ 23 | € 17 | € 16–€ 17 | |
| Mean microbiology tests and average costs PPY | 3.4 | 3.3–3.6 | 2.4 | 2.2–2.5 |
| € 44 | € 42–€ 47 | € 31 | € 29–€ 32 | |
| Mean immunology tests and average costs PPY | 4.6 | 4.4–4.8 | 3.2 | 3.0–3.4 |
| € 92 | € 88–€ 96 | € 64 | € 61–€ 67 | |
| Mean tests PPY | 70.3 | 69.2–71.7 | 50.7 | 49.4–51.7 |
| Total average tests PPY | € 195 | € 187–€ 202 | € 138 | € 131–€ 142 |
| Mean anal canal procedures and average costs PPY | 0.39 | 0.34–0.45 | 0.56 | 0.50–0.63 |
| € 155 | € 135–€ 179 | € 223 | € 199–€ 251 | |
| Mean Day care Hospital Dexascans and average costs PPY | 0.21 | 0.17–0.26 | 0.08 | 0.06–0.11 |
| € 13 | € 11–€ 17 | € 5 | € 4–€ 7 | |
| Mean Day care Hospital Fibroscans and average costs PPY | 0.05 | 0.03–0.08 | 0.05 | 0.03–0.07 |
| € 3 | € 2–€ 5 | € 3 | € 2–€ 5 | |
| Mean Imaging Procedures and average costs PPY | 0.28 (95%CI 0.24–0.33)–€ 11 (95%CI € 10–€ 13) | |||
| Mean procedures per PPY | 0.93 | 0.78–1.12 | 0.97 | 0.83–1.14 |
| Total annual cost procedures | €182 | €158–€214 | €242 | €215–€276 |
| Total annual cost of outpatient services | €1073 | €999–€1157 | €1158 | €1084–€1238 |
| Annual ARV cost | €7557 | |||
| Total annual outpatient costs including ARVs | €8430 | €8356–8514 | €8515 | €8441–8595 |
The annual cost PPY of outpatient visits increased by 12% from €696 (95%CI €654–€741) pre-EmERGE to €778 (95%CI €738–€820) post-EmERGE. The mean number of tests decreased between the two periods (Table 4), as did average costs by 29% (Table 5). The mean number of anal canal procedures increased resulting in a 33% increase of costs for these procedures, while costs for dexascans decreased and that for fibroscans remained the same between periods (Table 4).
Annual mean use and cost PPY HIV outpatient visits, tests and procedures, pre- and post-EmERGE implementation at the HC-IDIBAPS for participants used face-to-face outpatients only (F2F) and participants who use virtual and face-to-face clinics (V2F).
| F2F participants average annual cost clinic visits | €668 | 95%CI €630–709 | €594 | 95%CI €573–614 |
| F2F participants average costs tests & procedures | €290 | 95%CI €275–309 | €310 | 95%CI €290–331 |
| F2F participants total average annual outpatient cost | €958 | 95%CI €905–1018 | €904 | 95%CI €863–945 |
| V2F participants average annual outpatients visit costs | €28 | 95%CI €24–32 | €181 | 95%CI €163–203 |
| V2F participants average costs tests & Procedures | €87 | 95%CI €70–107 | €70 | 95%CI €56–87 |
| Total Average annual cost V2F participants | €115 | 95%CI €94–139 | €251 | 95%CI €219–290 |
| Total annual cost of outpatient services | €1073 | 95%CI €999–€1157 | €1158 | 95%CI €1084–€1238 |
The average annual costs of outpatient visits, tests and procedures increased by 8% across periods from €1073 (95%CI €999–€1157) to €1158 (95%CI €1084–€1238) PPY. The average annual cost of ARVs was €7557 and total average annual costs increased by 1% from €8430 (95%CI €8356–8514) to €8515 (95%CI €8441–8595) PPY (Table 4).
When comparing the 433 participants only attended F2F clinics, with those who started with the virtual service and then transferred to a face-to-face (V2F), the annual use and cost for participants that only used F2F services decreased by 5% from €958 (95%CI 905–1018) to €904 (95%CI 863–945; Table 5). Conversely, post-EmERGE 113 V2F participants were transferred to the F2F outpatient services (Table 3); cost of services for V2F participants increased by a factor of 2.2 from €115 (95%CI 94–139) pre- to €251 (95%CI 219–290) post-EmERGE.
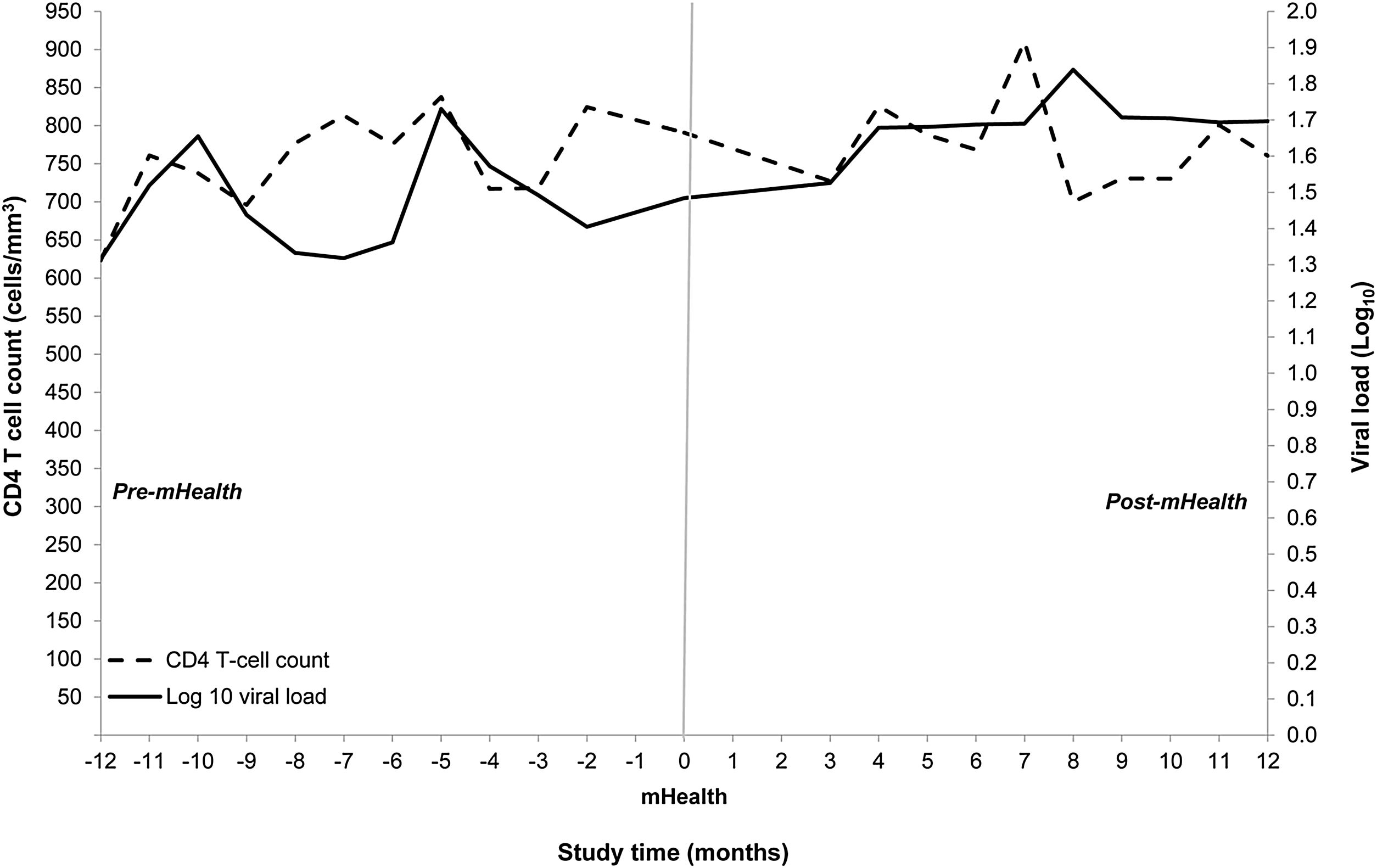
Primary and secondary outcomes pre- and post-mHealthMedian CD4 count at baseline was 759cells/mm3 (IQR 578–976cells/mm3) and CD4 counts did not differ significantly between periods. Viral load at baseline was less than 20copies/ml for all but four participants; the viral load of all participants was undetectable shortly after study entry and remained so during the study (Fig. 1).
EmERGE participants’ secondary outcome measure PAM-13, measure of patient activation, and their quality of life indicators based on PROQOL-HIV, did not change substantially over time (Table 6).
Median and IQR for PAM13and PROQOL-HIV Months 0 and 12, baseline – and post-EmERGE at the HC-IDIBAPS.
| PAM 13 | Month 0 – recruitment baseline | Month 12 Post-EmERGE | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| 70.2 | (58.1–80.9) | 67.8 | (58.1–77.7) | |
| PROQOL-HIV | Month 0 (Baseline) | Month 12 (Post-EmERGE) | ||||
|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | |
| Physical health and symptoms | 535 | 88.9 | 77.8–97.2 | 281 | 83.3 | 69.4–94.4 |
| Body change | 535 | 93.8 | 81.3–100.0 | 280 | 87.5 | 68.8–100.0 |
| Social relationships | 540 | 100.0 | 100.0–100.0 | 283 | 100.0 | 100.0–100.0 |
| Intimate relationships | 534 | 91.7 | 66.7–100.0 | 282 | 83.3 | 50.0–100.0 |
| Stigma | 540 | 50.0 | 25.0–87.5 | 282 | 50.0 | 25.0–87.5 |
| Emotional distress | 537 | 93.8 | 75.0–100.0 | 280 | 87.5 | 68.8–100.0 |
| Health concerns | 537 | 81.3 | 62.5–100.0 | 279 | 81.3 | 56.3–93.8 |
| Treatment impact | 533 | 87.5 | 80.0–95.0 | 277 | 85.0 | 77.5–92.5 |
The 8% increase in use and associated costs of HIV outpatient services at HC-IDIBAPS was related to the transfer of EmERGE participants from the virtual ICMID Daycare Office to the two F2F clinics. The participants who only used F2F clinics recorded a 5% reduction in use and cost of services. Similar reductions to those observed in the F2F population were, mutatis mutandis, to those observed in the other EmERGE clinics.32 The increased cost generated by V2F participants occurred because they were transferred during the second year to the more ‘expensive’ F2F.
The observed increase in some of the procedures also contributed to the rise in cost. During the first year of EmERGE, anal canal procedures were performed by staff who also had other duties. During the second year, dedicated staff were appointed, which increased the number of referrals for anal scans and the increased number of scans performed, increased expenditure. These changes, however, only minimally affect the conclusion of this analysis, that the implementation of the EmERGE Pathway was an efficient intervention in the HC-IDIBAPS.
Participants, including women and migrants, enjoyed having the EmERGE mHealth App and following the new Care Pathway. The App provided the participants with autonomy, as they became less dependent on going to the clinic for routine consultations. The test results function was the most important for participants.33
The majority of participants expressed trust in the privacy and security of the EmERGE platform and this was growing over time. Some privacy concerns remained especially among black and migrant women; they were anxious that the App could be seen on the phone by friends or family members, who then would ask questions.33
Participants remained medically stable and did not have substantive changes in their patient activation and quality of life measures.34 The before-and-after study provides a robust method for assessing the effect of the intervention and is a favoured method for health-technology assessments.35 The study is limited by the fact that a control-group was not constituted for the secondary outcome measures – PAM-13 and PROQOL-HIV.
The costs of ARVs used by EmERGE participants was €7557, within the 2017 price range for ARVs in Spain.36 ARVs were the main cost-driver of outpatient services for EmERGE participants and a switch to quality-assured and affordable generic ARVs could reduce these costs, although the use of generic drugs can raise other issues.37 Health care costs can be further reduced when using a single daily pill regimen,38 especially when using generic versions.
Most departments could be micro-costed, apart from the radiology and overhead departments. The HC-IDIBAPS overhead costing system was adopted but the accuracy of the fixed percentages and the extent they covered all overhead costs could not be established.22
This is the most recent Spanish cost-of-illness study for medically stable PLHIV; no other recent studies could be identified. A study from the Canary Islands reported an annual cost for asymptomatic PLHIV services of €10,351 in 200339; a second study, reported the cost for asymptomatic PLHIV to be €8548 at 2010 prices.40
The expansion of electronic health information systems highlights the need to protect the confidentiality and security of personal health information: at rest – on a phone or server(s) – and in-transit; such protection is of paramount importance.41 Guidance for protecting personal health information have been published15 and paper-based and electronic tools developed to investigate the existence and implementation of these guidelines at facility, data warehouse and national levels.42
The EmERGE study was successfully implemented in five different European countries, which demonstrated its applicability in different cultural settings.32 It also points to the benefits of including virtual appointments as part of outpatient managements for PLHIV.
Many published systematic reviews high-light the variable effectiveness of mHealth tools.16 Some reviews were HIV-specific3,4,43 whereas others reviewed mHealth tools for other chronic diseases.44,45 Most studies were performed in high-income countries, but the use of mHealth is increasingly promoted in low- and middle-income countries.46–51 However, some observations remain relevant today: “Most of the studies analysed were small scale, short term, pragmatic evaluations that added little to our knowledge of the costs and benefits that would be expected to result from the introduction of telemedicine services into routine clinical practice.16” EmERGE involved more participants across different health care systems and countries, but only involved medically stable PLHIV and follow-up was still only one year post-EmERGE implementation.
While efficiencies were achieved at HC-IDIBAPS, broader implementation of the EmERGE Pathway to all PLHIV are likely to lead to greater efficiencies; these savings can be used to address other needs within the clinic. Extending the use of the EmERGE Pathway should be systematically and closely monitored and implementation evaluated and costed.3,4,18,23 As this study focused on medically stable PLHIV, the services for managing PLHIV with more complex HIV disease or comorbidities need to be costed. Funding should be sought from relevant agencies to monitor and evaluate changes in service provision.
The use of mHealth tools has expanded during the Covid19 Pandemic for both acute and chronic diseases,52 including cancer and mental health services.7,53 This expansion is expected to continue; in 2019, the global mHealth market was valued at US$46 billion and is expected to reach $230 billion by 2027.54 The expansion of these tools should make tracking the use, cost, outcomes and impact of health services across facilities easier. Linking individual health information over time and place is part of the aim of Universal Health Coverage.55
Conflict of interestsThe authors declare that they have no conflict of interest.