Escherichia coli is a very infrequent cause of spontaneous community-acquired meningitis in adults (incidence lower than 0.1 cases per 100,000 adults per year).1–3 This organism usually reaches the meninges from a distant source, being a rare complication of a bloodstream infection.3 In general, the mortality rate of patients with Gram-negative bacillary meningitis is higher than that caused by Neisseria meningitidis,3 which is probably due to infections caused by especially virulent strains. Virulence factors including toxins, adhesins, lipopolysaccharides, different capsular types, and proteases are important for the invasion and dissemination of E. coli in the host.4 We report a case of meningitis in an adult caused by E. coli, and characterize the pathogenicity of the isolate in vitro and in vivo.
A 58-year-old man was admitted to the emergency department of our hospital with fever, dysuria and frequent urination. Therapy with amoxicillin-clavulanic acid was started, and after 24h of improvement, the patient developed a severe occipital headache and emetic syndrome, followed by hemodynamic instability and altered mental status. With the suspicion of meningoencephalitis, a lumbar puncture was performed obtaining a cloudy cerebrospinal fluid (CSF) with severe neutrophilic pleocytosis (>4000cells/μL), elevated proteins (3.8g/L) and low glucose (<0.02g/L). Empirical treatment with ceftriaxone and ampicillin was started. The patient was admitted to the ICU and required mechanical ventilation for 9 days. E. coli C1-7-LE, only resistant to cotrimoxazole, was isolated from blood cultures, urine, and CSF. At day 7, the axial computerized tomography showed diffuse edema without other complications. At day 8, a second CSF analysis showed a biochemical improvement: proteins and glucose concentrations were 0.94 and 0.43g/L, respectively, absence of bacteria in the Gram stain and absence of growth in the aerobic culture. At day 12, the patient was discharged to the infectious diseases ward. Due to persistent mutism and altered mental status, a magnetic resonance (MR) imaging was performed revealing the presence of multiple abscesses. Endovenous treatment with ceftriaxone was maintained, which improved the neurologic symptoms and the lesions in the MR. The patient was discharged from the hospital after 8 weeks of treatment.
We studied the pathogenicity of the E. coli C1-7-LE isolate causing the meningitis. MLST analysis showed that it belonged to the phylogroup B2.
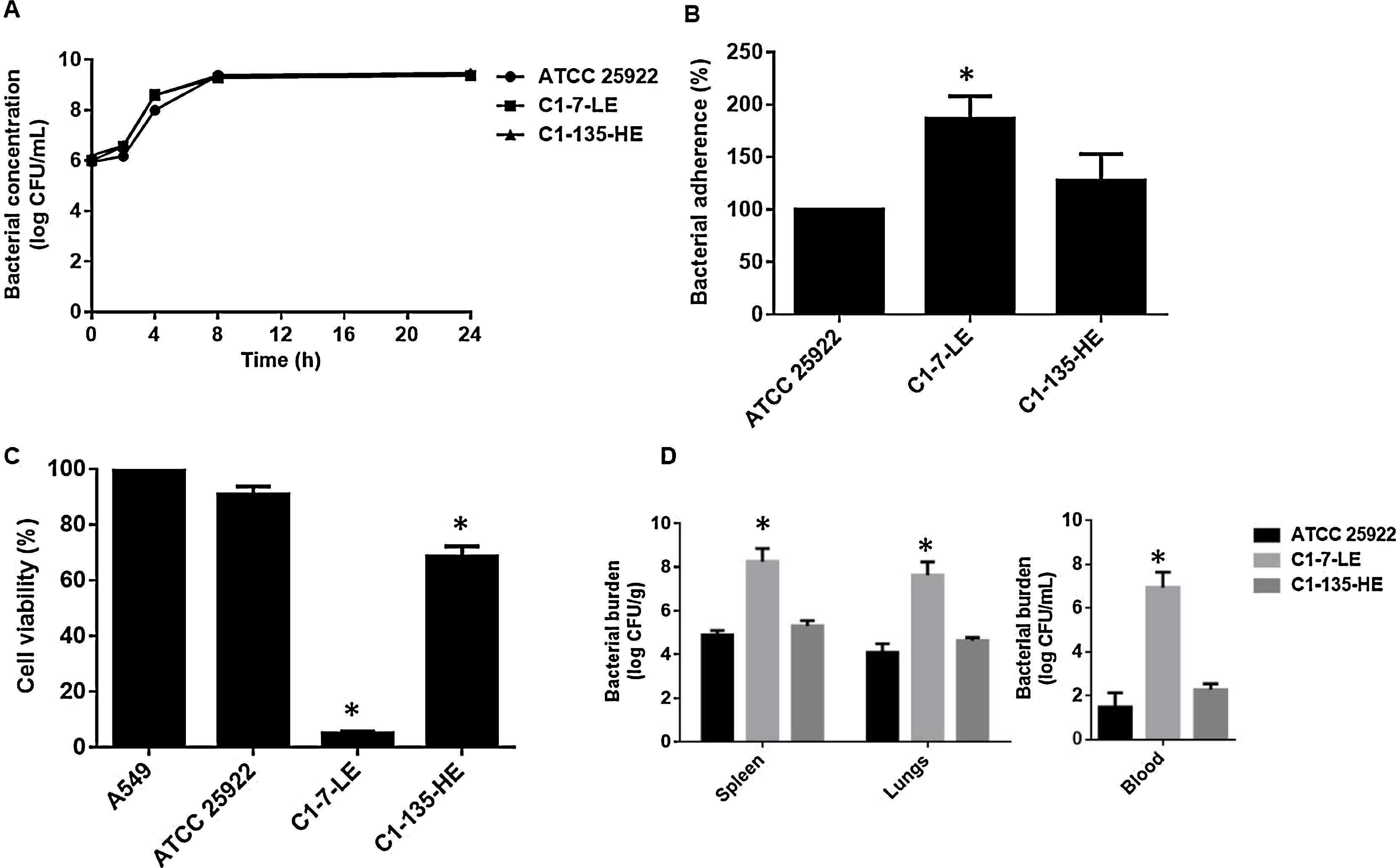
Two control strains were used for comparison: (i) – the reference strain E. coli ATCC 25922, belonging to phylogroup B2, and (ii) – a clinical E. coli strain C1-135-HE belonging to phylogroup A, isolated from a patient without meningitis but with similar clinical parameters that our meningitis case patient. In vitro bacterial growth showed that C1-7-LE had similar growth rate compared to both control strains (Fig. 1A). A bacterial adhesion assay was performed as described previously.5 C1-7-LE presented (approximately) an 87% and 28% of adherence increase to A549 cells when compared with ATCC 25922 (P=0.03) and C1-135-HE (P=0.29), respectively (Fig. 1B).
(A) Growth curve of E. coli C1-7-LE strain producing meningitis, and control strains E. coli ATCC 25922 and E. coli C1-135-HE in Mueller-Hinton broth for 24h. (B) Bacterial adherence assay in A549 cells of E. coli strains C1-7-LE, C1-135-HE, and ATCC 25922. Representative results of 3 independent experiments (mean±SEM by unpaired Student t-test P<0.05: *comparison between ATCC 25922 and C1-7-LE or C1-135-HE). (C) Viability of A549 cells after incubation with the 3 isolates for 6h. Representative results of 3 independent experiments (mean±SEM. by unpaired Student t-test P<0.05: *comparison between A549 cells and other groups). (D) Bacterial burden in spleen, blood and lungs after 6h of infection (inoculum of 7.3log10CFU/mL) with the 3 strains (mean±SEM. P<0.05: *comparison between ATCC 25922 and C1-7-LE or C1-135-HE strains).
In order to analyze the ability of the meningitis isolate to potentiate host cell death, we studied the viability of A549 cells in presence of both the C1-7-LE isolate and the control strains by the MTT assay as described previously.6 C1-7-LE was more cytotoxic than the control strains, reducing the A549 cell viability to 5.00±0.71% (P=0.006) at 6h, while the ATCC 25922 and C1-135-HE isolates reduced the cell viability to 90.75±3.00% (P=0.14) and 68.54±3.67% (P=0.01), respectively (Fig. 1C).
Finally, a previously characterized murine peritoneal sepsis model was used to assess the pathogenicity of the meningitis-producing E. coli in comparison with both control isolates.7 Spleen, blood and lungs showed higher bacterial concentrations in mice infected with the C1-7-LE isolate than those infected with ATCC 25922 and C1-135-HE as follows: 8.26±0.59, 6.95±0.70 and 7.63±0.61logCFU/g (C1-7-LE) vs. 4.9±0.21, 1.48±0.66 and 4.10±0.40logCFU/g, (P<0.05, ATCC 25922) and 5.21±0.24, 2.27±0.28 and 4.64±0.14logCFU/g (P<0.05, C1-135-HE) (Fig. 1D).
In summary, the meningitis-producing E. coli isolate presented higher adherence to host cells, was more cytotoxic and showed increased pathogenicity in a murine experimental model, characteristics that could explain the high virulence of this E. coli isolate in our case. Experimental studies are useful in order to evaluate whether some of the virulence factors may be involved in the pathogenesis of isolates causing severe infections.
This study was approved by the Ethic Committee of University Hospital Virgen del Rocío, Seville (approval no. 0023-N-16).
FundingThis study was supported by the Miguel Servet Tipo I Project grant, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad (CP15/00132). Y.S. is supported by the Subprograma Miguel Servet Tipo I, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spain (CP15/00132). R.A.M. is supported by the Subprograma Rio Hortega, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spain (CM14/00179).