Sexually transmitted infections (STIs) are common in our environment, and trends have been increasing in the last few years. Different methods for STIs diagnosis have been applied by microbiology laboratories over years, but real-time PCR has improved this process. Our objective was to evaluate VIASURE Sexually Transmitted Diseases Real-Time PCR Detection kit (CerTestBiotec, S.L.) comparing with the real-time PCR technique used in our laboratory (Allplex™ STI7 Essential Assay, Seegene) which was considered as reference assay.
MethodsA total of 948 samples from different sites (vaginal, endocervical, urethral, rectal, pharyngeal swabs and urine samples) were analyzed from July to September 2018.
ResultsA discordant result was obtained in 4.5% (43 samples). These discrepancies were mainly observed in threshold cycle (Ct) value next to the limit of detection. The k coefficient obtained shows a very high agreement between both methods with k values from 0.92 to 0.99.
ConclusionsVIASURE Sexually Transmitted Diseases Real-Time PCR Detection kit provides a very good correlation with Allplex STI7 and therefore, it's a good tool for the diagnostic of STIs. Positive results with Ct value obtained from 35 and low amplification signal should be applied with caution and should be interpreted based on the patient's clinical data.
Las infecciones de transmisión sexual (ITS) son frecuentes en nuestro entorno y con tendencia a aumentar. Las técnicas moleculares han mejorado su diagnóstico aportando sensibilidad y rapidez de resultados. Nuestro objetivo ha sido evaluar el nuevo kit de PCR a tiempo real VIASURE® Sexually Transmitted Diseases (CerTest Biotec, SL) comparándolo con la técnica de PCR a tiempo real empleada en nuestro laboratorio (AllplexTM STI7 Essential Assay, Seegene).
MétodosSe analizaron prospectivamente un total de 948 muestras de diferente localización (exudado vaginal, endocervical, uretral, rectal, faríngeo y orina) recibidas en nuestro laboratorio desde julio hasta septiembre de 2018.
ResultadosEn el 4,5% (43 muestras) se obtuvo un resultado discordante entre ambas técnicas. Estas discrepancias se observaron principalmente en ciclos próximos al límite de detección. El valor del coeficiente k osciló entre 0,92 a 0,99, mostrando una muy buena correlación entre técnicas.
ConclusionesVIASURE® Sexually Transmitted Diseases Real-Time PCR Detection kit es una buena herramienta para el diagnóstico de las ITS. Muestras con señal de amplificación en ciclos a partir de 35 y baja señal de fluorescencia, deben ser tratados con precaución e interpretarse en función de los datos clínicos del paciente.
Sexually transmitted infections (STIs) are common in our environment, and trends have been rising in the last few years.1,2 STIs compromise quality of life as well as sexual and reproductive health and further facilitate the spread of infections such as human immunodeficiency virus infection (HIV), mainly in men who have sex with men (MSM). This situation not only represents an important individual problem, but also a Public Health issue. The patient can present a curable acute infection such as urethritis, cervicitis, proctitis and genital ulcers but this can also lead to serious complications such as cervical cancer, ease of HIV infection, and pelvic inflammatory disease (PID), which is a major cause of infertility, ectopic pregnancy, and chronic pelvic pain. The main objectives are focused on controlling and decreasing STIs transmission, and to improve the diagnosis in accordance to the guidelines of the Center Disease Control (CDC)3 and World Health Organization.4
There is a STIs work group in Catalonia that suggests, evaluates and develops screening programs. Work group consultants are sexually transmitted diseases (STD) clinicians of primary service and sexual and reproductive healthcare departments, representative members of the Public Health department, members of the Catalonia Center for HIV/AIDS, employees of sexual education center specific to children, and clinicians from different hospitals, such as microbiologist, dermatologist, infectologist and gynecologist. They make recommendations for the laboratory diagnosis and establish standard operating procedures to collect and process specimens, counsel and treat patients. Objectives of this group are to produce more rapid and accurate diagnosis, and to treat the patient as soon as possible ensuring quality. Furthermore, other efforts include having a critical role in prevention and this is why a screening program was implemented for all active individuals younger than 25 and everyone aged >25 with risk factors (e.g., those who have a new sexual partner or multiple partners).5
Most of the Clinical Microbiology laboratories use different methods for STIs diagnosis.6 Culture has all along been the reference standard for some pathogens but can have false negative results due to difficulties in maintaining the viability of organism during transport and storage or previous treatment. Serological tests are adequate for epidemiological purposes but could not distinguish between active and past infections in some cases. Due to nucleic acid amplification tests (NAATs), diagnosis has been improved. Molecular techniques provide faster results, more sensibility, do not require viable organism and additionally, they are the only available method for the detection of some microorganisms.7–10
VIASURE Sexually Transmitted Diseases panel is a qualitative multiplex assay of real-time PCR able to detect and to identify 7 microorganisms related to genital pathology and/or STIs. Our objective was to evaluate VIASURE Sexually Transmitted Diseases Real-Time PCR Detection kit comparing with the real-time PCR technique used in our laboratory (Allplex™ STI7 Essential Assay, Seegene) which was considered as reference assay. Both assays detect Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma genitalium and Mycoplasma hominis.
Material and methodsPatients and samplesThis was a prospective study conducted from July to September 2018 at the Microbiology department of Laboratory Clinic Metropolitan Nord in Badalona (Barcelona, Spain). This laboratory receives samples from the North Metropolitan area that includes 106 primary health care facilities and 6 sexual and reproductive health centers; corresponding to more than 1,400,000 inhabitants. In our molecular biology Department next to 20,000 samples coming from different sites of symptomatic and asymptomatic patients are analyzed annually for STI diagnosis.
A total of 948 samples from different locations were included in this study: endocervical (n=492), vaginal (n=327), urethral (n=20), rectal (n=4), pharyngeal (n=2) swabs and urine samples (n=103). Samples were collected with flocked swabs in Amies liquid transport medium (Deltalab S.L, Barcelona, Spain) optimal for PCR assays except urine samples that were collected in clear containers of polypropylene. Specimens were received at the laboratory within 24h after collection, and were stored at 4°C until they were processed, which has to be upon reception within 48h.
Molecular detection and principle of the procedureAll samples were simultaneously analyzed for Viasure and Allplex STI7. According to each manufacturer, urine specimens and genital swab specimens analysis are only permitted. Both methods detect the main microorganisms that cause STIs: Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma genitalium and Mycoplasma hominis.
Nucleic acid extraction was performed using the STARLET platform (Hamilton Company, Reno, EEUU) following protocol established according to the manufacturer's instructions. The real-time PCR termocycler used to amplification process was provided by each company: CFX96™ Real-Time PCR Detection System (Bio-Rad) and DTprime Real-time Detection Thermal Cycler (DNA-Technology).
VIASURE Sexually Transmitted Diseases Real-Time PCR Detection Kit is based on 5′ exonuclease activity of DNA polymerase. During DNA amplification, this enzyme cleaves the probe bound to the complementary DNA sequence, separating the quencher dye from the reporter. This reaction generates an increase in the fluorescent signal which is proportional to the quantity of target template. This fluorescence could be measured on real-time PCR platforms. This kit used to the amplification of a conserved region of the T. vaginalis-specific 2-kb repeated sequence, urease gene (U. urealyticum and U. parvum), yidC gene (M. hominis), porA and Opa genes (N. gonorrhoeae), a region within orf2 of the chlamydial plasmid (C. trachomatis) and MgPa adhesion gene (M. genitalium), using specific primers and fluorescent-labeled probe.
Allplex™ based on Seegene's innovative MuDT™ technology which allows to provide multi-Ct (threshold cycle) values in a single fluorescence channel without melting curve analysis on real-time PCR instrument.
Allplex STI7 is able to detect the multiple targets in a single reaction with high sensitivity, specificity, and reproducibility. However, VIASURE includes two kinds of strips and each one corresponds to one different master mix. The first strip contains the multiplex reaction mix for the detection C. trachomatis, N. gonorrhoeae and M. genitalium. The second strip contains the reaction mix for the detection T. vaginalis, U. parvum, U. urealyticum and M. hominis.
Leftover samples and nucleic acids extracts were conserved at -80°C to analyze any possible discrepancies. Discrepancies between methods were retested by a third real-time PCR assay; the FTD Urethritis plus Test (Fast-track Diagnostics, Luxembourg).
Interpretation of results and statistical analysisFor the detection and for the data analysis was used the software designed by each company. Results were classified as a true positive or true negative when both assays showed an identical result and as a discrepant result when the test was positive by one method but negative by the another assay.
Allplex STI7 assay was considered our reference assay to calculate the sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV) and kappa index (k) for each microorganism. The results were calculated through the web-based epidemiologic and statistical calculator OpenEpi, www.openepi.com (Emory University, Atlanta, USA). The level of agreement between both assays was calculated by means of Kappa statistics.
Ethic statementEthical approval from the Ethics Committee Research of Germans Trias i Pujol University Hospital Ethics Committee was obtained (PI-20-094) and the need for informed consent was waived.
ResultsA total of 948 samples were analyzed by two multiplex real-time PCR. Both assays have a comparable hands-on time and time to result (approximately 3.5h). Furthermore, both companies have developed software to interpret the results easily.
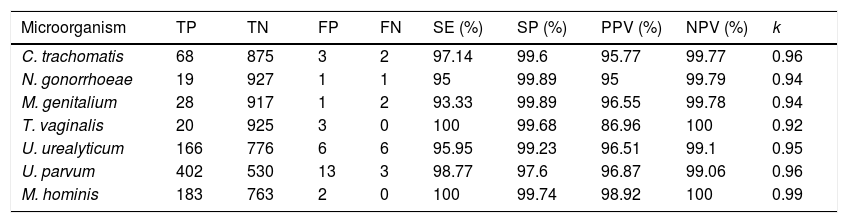
Fully concordance was obtained in 905 (95.5%) specimens and discordant results were obtained in 4.5% (43 samples). Data for sensitivity, specificity, negative predictive value, positive predictive value and kappa index for each microorganism are shown in Table 1. The k coefficient shows a very high agreement between both methods with k values from 0.92 to 0.99 depending on microorganism tested.
Performance of VIASURE kit in comparison with Allplex STI7.
| Microorganism | TP | TN | FP | FN | SE (%) | SP (%) | PPV (%) | NPV (%) | k |
|---|---|---|---|---|---|---|---|---|---|
| C. trachomatis | 68 | 875 | 3 | 2 | 97.14 | 99.6 | 95.77 | 99.77 | 0.96 |
| N. gonorrhoeae | 19 | 927 | 1 | 1 | 95 | 99.89 | 95 | 99.79 | 0.94 |
| M. genitalium | 28 | 917 | 1 | 2 | 93.33 | 99.89 | 96.55 | 99.78 | 0.94 |
| T. vaginalis | 20 | 925 | 3 | 0 | 100 | 99.68 | 86.96 | 100 | 0.92 |
| U. urealyticum | 166 | 776 | 6 | 6 | 95.95 | 99.23 | 96.51 | 99.1 | 0.95 |
| U. parvum | 402 | 530 | 13 | 3 | 98.77 | 97.6 | 96.87 | 99.06 | 0.96 |
| M. hominis | 183 | 763 | 2 | 0 | 100 | 99.74 | 98.92 | 100 | 0.99 |
TP, true positives; TN, true negatives; FP, false positives; FN, false negatives; SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value, κ, Kappa coefficient.
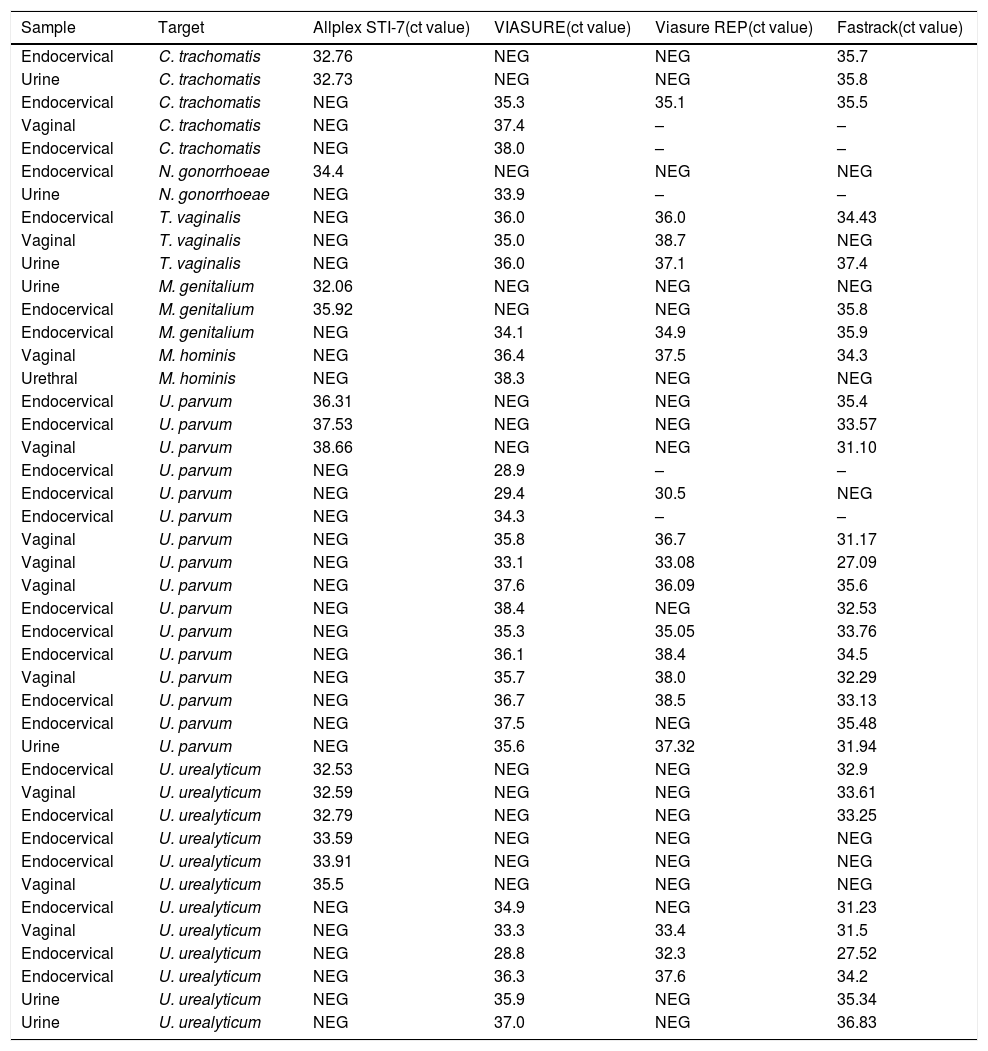
The discordant samples were: 24 endocervical swabs (4.9%), 11 vaginal swabs (3.4%), 7 urine samples (6.8%) and 1 urethral swab (5%). Three endocervical swabs (two false-positive and one false-negative), one vaginal swab (false-positive) and urine sample (false-negative) were discordant for C. trachomatis and for N. gonorrhoeae were discordant one endocervical swab (false-negative) and one urine sample (false-positive). T. vaginalis detection was discordant in one endocervical and one vaginal swab (both false-positive) and one urine sample (false-positive). M. genitalium detection was discordant in two endocervical swabs (false-negative and false-positive) and one urine sample (false-negative). One vaginal swab and one urethral swab were false-positive for M. hominis. Ten endocervical swabs (two false-negative and eight false-positive), five vaginal swabs (four false-positive and one false-negative) and one urine sample (false-positive) were discrepant samples for U. parvum. Seven endocervical swabs (four false-negative and three false-positive), three vaginal swabs (two false-negative and one false-positive) and two urine samples (false-positive) were discrepant for U. urealyticum.
Discordant results were retested with VIASURE assay and another real-time PCR assay (FTD Urethritis plus, Fast-Track Diagnostics). Five samples were impossible retested due to not available neither sample nor eluted. The discrepancies were mainly observed in Ct value next to limited detection and low fluorescence signal. The analysis with FTD Urethritis plus assay showed a 24% overall agreement (9/38) with Allplex STI assay and a 68% overall agreement (26/38) with the VIASURE assay. It should be noted that with the repetition of 5 samples with VIASURE, the results changed. A detailed description of the discrepant results is shown in Table 2.
Discordant results detected by Viasure and Allplex STI-7 assay.
| Sample | Target | Allplex STI-7(ct value) | VIASURE(ct value) | Viasure REP(ct value) | Fastrack(ct value) |
|---|---|---|---|---|---|
| Endocervical | C. trachomatis | 32.76 | NEG | NEG | 35.7 |
| Urine | C. trachomatis | 32.73 | NEG | NEG | 35.8 |
| Endocervical | C. trachomatis | NEG | 35.3 | 35.1 | 35.5 |
| Vaginal | C. trachomatis | NEG | 37.4 | – | – |
| Endocervical | C. trachomatis | NEG | 38.0 | – | – |
| Endocervical | N. gonorrhoeae | 34.4 | NEG | NEG | NEG |
| Urine | N. gonorrhoeae | NEG | 33.9 | – | – |
| Endocervical | T. vaginalis | NEG | 36.0 | 36.0 | 34.43 |
| Vaginal | T. vaginalis | NEG | 35.0 | 38.7 | NEG |
| Urine | T. vaginalis | NEG | 36.0 | 37.1 | 37.4 |
| Urine | M. genitalium | 32.06 | NEG | NEG | NEG |
| Endocervical | M. genitalium | 35.92 | NEG | NEG | 35.8 |
| Endocervical | M. genitalium | NEG | 34.1 | 34.9 | 35.9 |
| Vaginal | M. hominis | NEG | 36.4 | 37.5 | 34.3 |
| Urethral | M. hominis | NEG | 38.3 | NEG | NEG |
| Endocervical | U. parvum | 36.31 | NEG | NEG | 35.4 |
| Endocervical | U. parvum | 37.53 | NEG | NEG | 33.57 |
| Vaginal | U. parvum | 38.66 | NEG | NEG | 31.10 |
| Endocervical | U. parvum | NEG | 28.9 | – | – |
| Endocervical | U. parvum | NEG | 29.4 | 30.5 | NEG |
| Endocervical | U. parvum | NEG | 34.3 | – | – |
| Vaginal | U. parvum | NEG | 35.8 | 36.7 | 31.17 |
| Vaginal | U. parvum | NEG | 33.1 | 33.08 | 27.09 |
| Vaginal | U. parvum | NEG | 37.6 | 36.09 | 35.6 |
| Endocervical | U. parvum | NEG | 38.4 | NEG | 32.53 |
| Endocervical | U. parvum | NEG | 35.3 | 35.05 | 33.76 |
| Endocervical | U. parvum | NEG | 36.1 | 38.4 | 34.5 |
| Vaginal | U. parvum | NEG | 35.7 | 38.0 | 32.29 |
| Endocervical | U. parvum | NEG | 36.7 | 38.5 | 33.13 |
| Endocervical | U. parvum | NEG | 37.5 | NEG | 35.48 |
| Urine | U. parvum | NEG | 35.6 | 37.32 | 31.94 |
| Endocervical | U. urealyticum | 32.53 | NEG | NEG | 32.9 |
| Vaginal | U. urealyticum | 32.59 | NEG | NEG | 33.61 |
| Endocervical | U. urealyticum | 32.79 | NEG | NEG | 33.25 |
| Endocervical | U. urealyticum | 33.59 | NEG | NEG | NEG |
| Endocervical | U. urealyticum | 33.91 | NEG | NEG | NEG |
| Vaginal | U. urealyticum | 35.5 | NEG | NEG | NEG |
| Endocervical | U. urealyticum | NEG | 34.9 | NEG | 31.23 |
| Vaginal | U. urealyticum | NEG | 33.3 | 33.4 | 31.5 |
| Endocervical | U. urealyticum | NEG | 28.8 | 32.3 | 27.52 |
| Endocervical | U. urealyticum | NEG | 36.3 | 37.6 | 34.2 |
| Urine | U. urealyticum | NEG | 35.9 | NEG | 35.34 |
| Urine | U. urealyticum | NEG | 37.0 | NEG | 36.83 |
Ct, cycle threshold; –, neither sample nor eluted available.
In the current study, the performance of a new multiplex real-time PCR assay (VIASURE Sexually Transmitted Diseases panel) was compared with the routine real-time PCR used in our laboratory (Allplex™ STI7 Essential Assay).
C. trachomatis and N. gonorrhoeae are the major causative agents of urethritis and cervicitis.11,12 In our study, the degree of concordance was higher between both assays (0.96 vs 0.94). Unfortunately, due to the lack of sample and eluted, it was impossible to retest the discrepancies obtained between two samples to C. trachomatis and one sample to N. gonorrhoeae.
It is important to mention that C. trachomatis variants have been described.13–15 Diverse modifications have been detected in regions used to NAATs, such as targets within C. trachomatis cryptic plasmid.13,15 VIASURE uses region orf2 within the chlamydial plasmid. In this region, mutations have not been described. A single-target, usually within the plasmid, is being used for chlamydia detection but our opinion, and other authors,7,15 is for molecular diagnostics should be used dual-target; within the plasmid and the chromosome, to avoid a false-negative.15
Trichomoniasis is also a sexually transmitted infection, more frequent in women than men, and the prevalence in MSM is very low. T. vaginalis infection causes urethritis, epididymitis, or prostatitis and, in infected women vaginal discharge. The degree of concordance was the lowest between both assays (0.92).
M. genitalium has become an emergent pathogen as a cause of male nongonococcal urethritis and in women it is associated with cervicitis and PID. Besides, M. genitalium has been found in different sites in both women and men and often in asymptomatic individuals. The need for treatment in asymptomatic individuals is controversial due to the rapid detection of macrolide resistance strains following a treatment.16,17 In fact, the guidelines strongly recommend that if NAAT diagnosis is available, macrolide resistance should be performed18 to avoid dissemination multidrug-resistant M. genitalium strains. Currently, treatment is only recommended in symptomatic patients and their current sexual partners.18 The degree of concordance was higher between both assays (0.94).
The major discrepancies were observed in the detection of U. parvum and U. urealyticum. But these bacteria and also M. hominis are isolated from the genital tract in both men and women, and therefore, asymptomatic carriage is common. U. urealyticum has been associated with urethritis in men and M. hominis has been associated with bacterial vaginosis and PID, but both with high titers. Only in these cases, when typical agents of STI have been excluded, the patient should be treated. Hence, routine screening is not recommended.19 The extensive testing and subsequent treatment could select antimicrobial resistance in these microorganisms.20
According to the results obtained with FTD Urethritis plus assay, the sensitivity and specificity of VIASURE assay was higher than Allplex STI7. One important difference between assays is the reaction mix. VIASURE, and FTD Urethritis plus assay, use two master mix to detect the 7 targets, meanwhile Allplex uses a single tube. Multiplex format could be less sensitive. Besides, the current study has some limitations. On the one hand, the lack of repetition of the analyses by the Allplex assay. When the fluorescence signal is low and the detection in Ct is close to the limits of detection, the results are not trustworthy. In our study, the results of 5 samples retested by VIASURE assay were different. And on the other hand, the limited number of samples from extragenital sites, such as the rectum and pharynx. After this study, the lack of T. vaginalis specificity with Allplex STI7 assay in pharyngeal swab was detected in our laboratory due to similar genetic identify between Trichomonas species. This finding has been documented by other authors.7
As a conclusion, the implementation in routine laboratory of new and faster molecular techniques is a current necessity as a consequence to important problem public health due to STIs. VIASURE Sexually Transmitted Diseases Real-Time PCR Detection kit provides a very good correlation with Allplex STI7 (Kappa index>0.90), as it is also user-friendly, sample-in, answer-out and therefore, an excellent tool for the diagnostic of STIs. Positive results with Ct value obtained from 35 and low amplification signal should be applied with caution and should be interpreted based on the patient's clinical data.
Conflict of interestThe authors declare no conflicts of interest.
We thank CerTestBiotec S.L. for providing the reagents and instrument used in this study.