The aim of this study was to identify multi-drug resistance (MDR) in the main enterobacteriaceae implicated in urinary tract infections (Escherichia coli and Klebsiella pneumoniae) from both, community and hospitalized patients and to analyze the evolution over a 12-year period.
MethodsMicrob Dynamic software was used to analyze the microbiology laboratory database and a chi square test was applied to compare differences in group proportions and to determine the linear trend over 12 years in three different periods: 2003–2006, 2007–2010, 2011–2014. We chose amoxicillin, gentamicin, ciprofloxacin and trimethoprim–sulphamethoxazole as MDR markers.
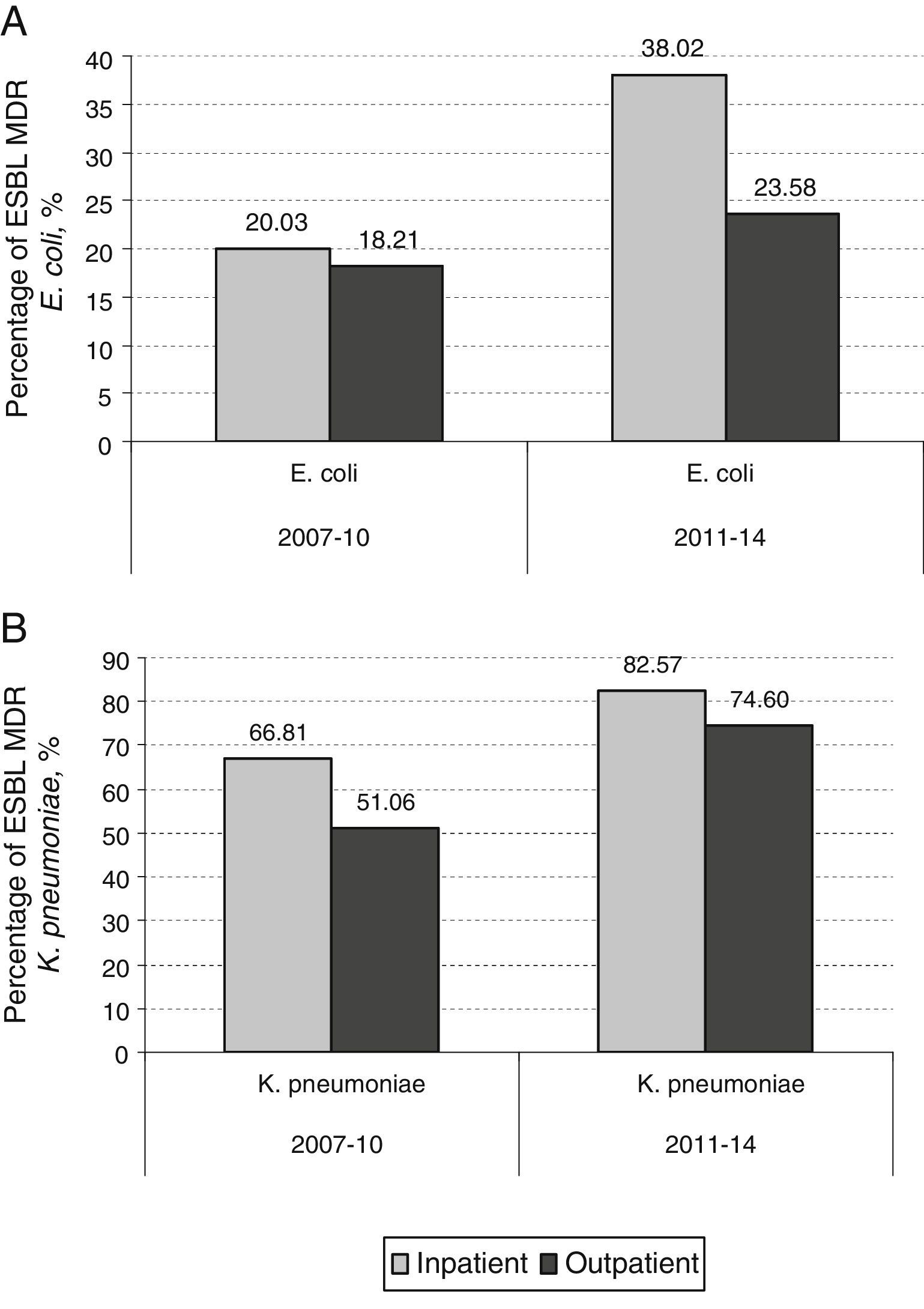
ResultsA total of 39,980 positive urine samples were analyzed, 34,564 (3786 from hospitalized patients and 30,778 from non-hospitalized patients) E. coli isolates, and 5,422 (774 from hospitalized patients and 4,648 from non-hospitalized patients) K. pneumoniae isolates. The prevalence of UTI due to MDR E. coli and MDR K. pneumoniae significantly increased in the period studied, both in hospitalized and outpatients. A higher percentage of MDR E. coli (5.89% in 2007–2010 versus 8.18% in 2011–2014) and MDR K. pneumoniae (2.38% in 2007–2010 versus 9.35% in 2011–2014) was evident and maintained constant over time in hospitalized patients in comparison to non-hospitalized ones. Infection due to MDR ESBL-producing E. coli and K. pneumoniae increased significantly during the last 8 years in both, hospitalized (20% versus 38% and 66.8% versus 82.6%, respectively) and non-hospitalized patients (18.2% versus 23.6% and 51% versus 74.6%, respectively).
ConclusionsThis study includes data of a large sample size of urinary strains isolated over a 12 year period and demonstrates that MDR is an increasing phenomenon of particular importance in the main UTI-causing species.
El objetivo principal de este estudio fue identificar multirresistencia a antibióticos (multi-drug resistance [MDR]) en las principales enterobacterias implicadas en infecciones del tracto urinario (ITU) (Escherichia coli y Klebsiella pneumoniae) procedentes de pacientes hospitalizados y ambulatorios, y analizar su evolución durante un periodo de 12años.
MétodosSe eligieron como marcadores de MDR amoxicilina, gentamicina, ciprofloxacino y trimetoprim-sulfametoxazol. Se realizó un tratamiento estadístico por chi cuadrado de los resultados obtenidos de nuestra base de datos y se analizó la tendencia lineal de la MDR en 3 periodos de 4 años: 2003-2006, 2007-2010 y 2011-2014.
ResultadosSe analizaron un total de 39.980 muestras de orina con cultivo positivo para E.coli (3.786 de pacientes hospitalizados y 30.778 de pacientes ambulatorios) y 5.422 con cultivo positivo para K.pneumoniae (774 de pacientes hospitalizados y 4.648 de pacientes ambulatorios). La prevalencia de ITU debida a MDR E.coli y MDR K.pneumoniae aumentó significativamente en el periodo estudiado, tanto en pacientes hospitalizados como en pacientes ambulatorios, observándose un mayor porcentaje de MDR E.coli (5,89% en 2007-2010 versus 8,18% en 2011-2014) y MDR K.pneumoniae (2,38% in 2007-2010 versus 9,35% en 2011-2014) en pacientes hospitalizados. La infección debida a MDR E.coli y K.pneumoniae productoras de β-lactamasas de espectro extendido (BLEA) aumentó también de forma significativa durante los últimos 8años, tanto en pacientes hospitalizados (20% versus 38% y 66,8% versus 82,6%, respectivamente) como en los no hospitalizados (18,2% versus 23,6% y 51% versus 74,6%, respectivamente).
ConclusionesEn este estudio se demuestra que la MDR es un fenómeno en aumento de particular importancia en las principales enterobacterias implicadas en ITU.
The increasing rate of antibiotic resistance in uropathogens, especially in Escherichia coli and Klebsiella pneumoniae, as the most common etiologic agents of urinary tract infections (UTI), leads to difficulties in choosing adequate empirical therapy and achieving treatment success.
Multiple studies regarding antibiotic susceptibility in uropathogens have been published in medical literature,1–4 but only a few studies have addressed the multidrug resistance (MDR) behaviour.5–7 According to a group of international experts from European Centre for Disease Prevention (ECDC) and Centers for Disease Control and Prevention (CDC) a standardized international definition for MDR was created. MDR is defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories.8
MDR bacteria are more usually associated with nosocomial infections. A significant part of nosocomial infections are diagnosed as UTI (more than 30%) and a considerable part of the antimicrobial agents used in hospitals is dedicated for their treatment.9 However, the vast majority of UTI come from outpatients, the most common being uncomplicated cystitis (infection of bladder) in women. The 2010 guidelines of the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases recommend for empirical treatment of acute uncomplicated cystitis trimethoprim–sulphamethoxazole (if the local resistance is less than 20%), nitrofurantoin, fosfomycin trometamol and pivmecillinam (as first-line therapy). Fluoroquinolones may be used only as alternative therapy and their use must be avoided to treat uncomplicated cystitis whenever possible.10 MDR bacteria at the community level has increased, making the treatment of infections more difficult and requiring, in some cases, intravenous therapy because of the lack of oral options.11
Several factors contribute to the increasing spread of MDR to antimicrobials. The overuse and misuse of antimicrobials inhuman medicine, in veterinary and in agriculture represent, among others, are some of the main factors involved in the selective pressure.
In addition, MDR in urine isolates from the community is often associated with the presence of multiple extended-spectrum-β-lactamases (ESBL) genes, as well as aminoglycoside and quinolone resistance genes.12
This study aims to identify MDR in the main enterobacteriaceae implicated as etiologic agents in UTI (E. coli and K. pneumoniae) from both, community and hospitalized patients, and to analyze the evolution over a 12-year period.
MethodsStudy population and bacterial isolatesA 12-year (2003–2014) retrospective study was conducted in both hospitalized and non-hospitalized patients suspected of UTI at the Hospital Universitario de Getafe (Madrid). The hospital has 413–600 operational beds and an attended population of 211,000–260,000 inhabitants (depending on the year). During the period of the study, each urine sample was cultured using a 1μL calibrated loop in cysteine lactose electrolytes deficient (CLED) agar and incubated at 37°C for 18–24h. A growth of >104 colony-forming units (CFU) per mL of one type of organism were considered as a positive result. Identification and antibiotic susceptibilities were performed using a microdilution system (WIDER, Franscico Soria Melguizo, SA).
Microb Dynamic software (Francisco SoriaMelguizo, SA) was used to analyze the microbiology laboratory database. It provides analysis of antimicrobial susceptibility and resistance test results according to the European Committee for Antimicrobial Susceptibility Testing (EUCAST) 2015 (version 5.0) clinical breakpoints (www.eucast.org). The software was used to determine the prevalence of both MDR E. coli and K. pneumoniae. In order to avoid distortions in the final results, isolates from the same patient with different antibiotic susceptibility patterns were considered as different while isolates showing the same pattern were considered as unique.
Statistical analysisA chi square test was applied to compare differences in group proportions in three different periods: 2003–2006, 2007–2010 and 2011–2014. Chi square test for trend was used to determine whether there was a statistically significant linear trend over the period of the study. A p-value <0.05 was considered as statistically significant. All statistical analyses were performed using Epi Info v. 7 (CDC, Atlanta, USA).
Antimicrobial susceptibilityWe determined the proportion of isolates resistant to at least the antimicrobials agents that we chose as MDR markers (amoxicillin, gentamicin, ciprofloxacin and trimethoprim–sulphamethoxazole) according to their clinical antimicrobial MIC breakpoints: amoxicillin (MIC >8mg/L), ciprofloxacin (MIC >1mg/L), trimethoprim–sulphamethoxazole (MIC >4/76mg/L) and gentamicin (MIC >4mg/L). Amoxicillin is not considered in K. pneumoniae because of its intrinsic resistance. We also determined the global prevalence of ESBL producing E. coli and K. pneumoniae (MDR and non-MDR) and the proportion of both, MDR E. coli and MDR K. pneumoniae isolates harbouring ESBL. Positive results derived from ESBL producing enterobacteriaceae isolates are routinely introduced in the database when phenotypic studies are developed in the suspected ESBL producers.
ResultsIn total, the laboratory counted 39,980 positive urine samples over the 12-year period. 34,564 positive urine samples (3786 from hospitalized patients and 30,778 from non-hospitalized patients) had E. coli as the infecting organism and 5,422 positive urine cultures (774 from hospitalized patients and 4,648 from non-hospitalized patients) had K. pneumoniae as the etiological organism.
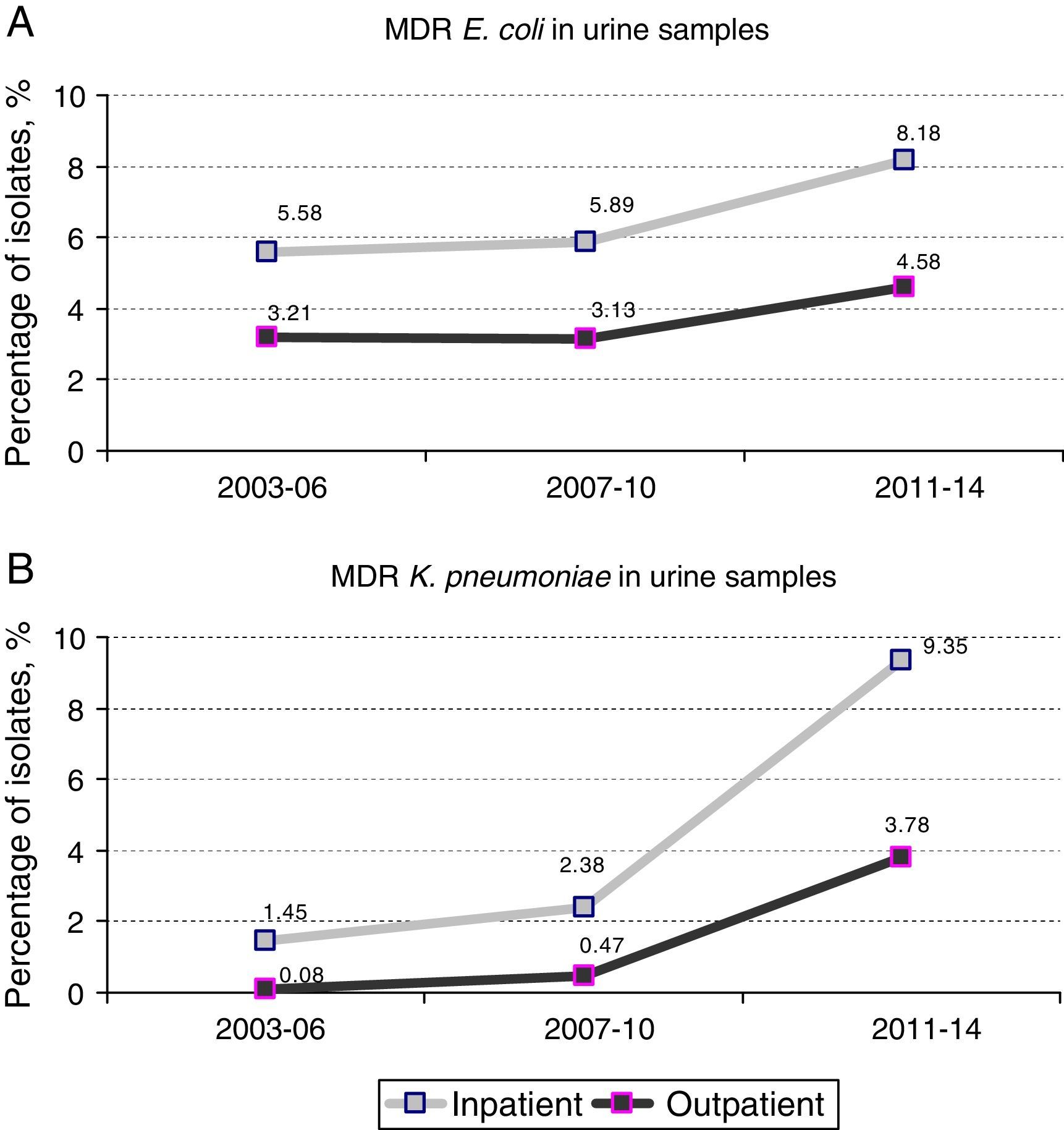
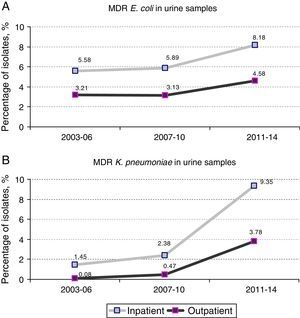
Prevalence and evolution of MDR profilesThe prevalence of UTI due to MDR E. coli (resistance at least to amoxicillin, ciprofloxacin, gentamicin and trimethoprim–sulphamethoxazole) in hospitalized patients increased from 5.89% [70 out of 1,188 isolates] in the period 2007–2010 to 8.18% [79 out of 966 isolates] in the period 2011–2014 (1.4-fold increase) (p=0.038). Similar results were observed in the same period when outpatient population was analyzed: 3.13% [332 out of 10,615 isolates] versus 4.58% [456 out of 9,947 isolates], respectively (p<0.001). No significant differences were observed between the first and the second periods of the study (2003–2006 and 2007–2010) (Fig. 1A).
However, the prevalence of UTI caused by MDR K. pneumoniae (resistance at least to ciprofloxacin, gentamicin and trimethoprim–sulphamethoxazole) suffered a more drastic change between 2007–2010 and 2011–2014. Fig. 1B shows a 4-fold increase in hospitalized patients (2.38% [6 out of 252 isolates] in 2007–2010 versus 9.35% [23 out of 246 isolates] in 2011–2014; p<0.001). This change in prevalence was even more pronounced (8-fold increase) when outpatient population was analyzed (0.47% [8 out of 1,689 isolates] in 2007–2010 versus 3.78% [67 out of 1,774 isolates] in 2011–2014; p<0.001) A slight but not significant increase was also observed between the first and second periods (2003–2006 versus 2007–2010) in both hospitalized and non-hospitalized patients (1.45% [4 out of 276 isolates] versus 2.38% [6 out of 252 isolates] and 0.08% [1 out of 1,185 isolates] versus 0.47% [8 out of 1,689 isolates] respectively) (Fig. 1B).
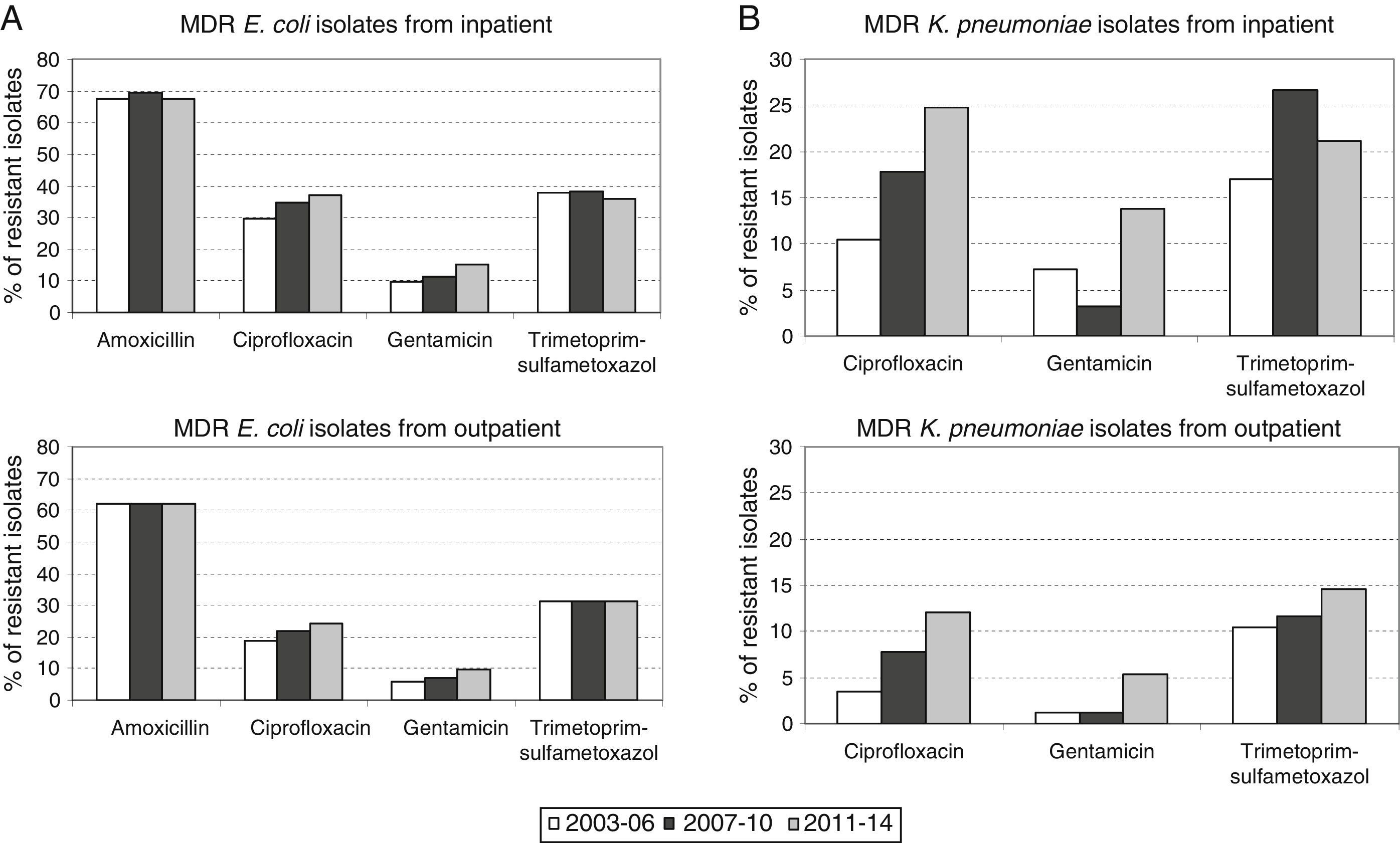
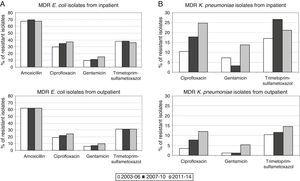
Therefore, higher percentages of MDR E. coli in hospitalized patients with respect to outpatients (1.8-fold increase in all the time periods) were evident and maintained constant over time (Fig. 1A). Similar results were observed in the percentage of MDR K. pneumoniae isolates, but the differences with respect to the non-hospitalized patients were more marked (>1.8-fold increase in all the time periods) (Fig. 1B). It is important to note that the increase observed in the prevalence of MDR E. coli isolates is mainly due to the individual resistance to ciprofloxacin and gentamicin regardless of the patient hospitalization, while individual resistance to amoxicillin and trimethoprim–sulphamethoxazole remained stable during the period of the study (Fig. 2A).
By contrast, the increase in prevalence of MDR K. pneumoniae isolates is due to the individual resistance to ciprofloxacin, gentamicin and trimethoprim–sulphamethoxazole (Fig. 2B).
ESBL contribution to the MDR profileThe global prevalence of UTI due to ESBL producing E. coli in hospitalized patients increased from 8.1% in the period 2003–2006 to 10.3% in the period 2011–2014 (1.27 fold-increase) while this increase in prevalence was more marked when non-hospitalized patients were analyzed (2.7% in 2003–2006 versus 5.7% in 2011–2014; 2 fold-increase).
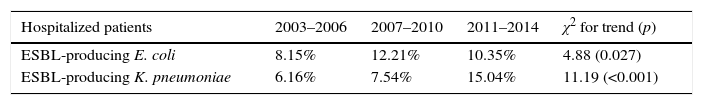
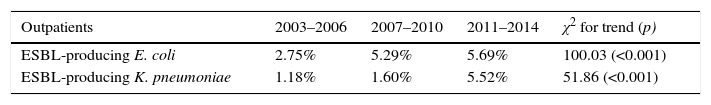
However, the rise in global prevalence of UTI due to ESBL producing K. pneumoniae in both, hospitalized and outpatients was more pronounced (6.1% in 2003–2006 versus 15% in 2011–2014; 2.4 fold-increase and 1.1% in 2003–2006 versus 5.5% in 2011–2014; 5 fold-increase, respectively). Table 1 show an analysis for linear trend in ESBL-producing E. coli and ESBL-producing K. pneumoniae in hospitalized patients (Table 1A) and non-hospitalized patients (Table 1B) over the period of the study.
Percentage of global ESBL-producing E. coli (A) and K. pneumoniae (B) urinary isolates from both, hospitalized patients and outpatients over the periods of the study (2003–2006, 2007–2010, and 2011–2013), and chi square test for trend. A p-value<0.05 was considered as statistically significant.
| Hospitalized patients | 2003–2006 | 2007–2010 | 2011–2014 | χ2 for trend (p) |
|---|---|---|---|---|
| ESBL-producing E. coli | 8.15% | 12.21% | 10.35% | 4.88 (0.027) |
| ESBL-producing K. pneumoniae | 6.16% | 7.54% | 15.04% | 11.19 (<0.001) |
In order to determine the contribution of ESBL in the MDR phenotype of both K. pneumoniae and E. coli, Microb Dynamic software was used to analyze the percentage of MDR isolates harbouring an ESBL.
The results show that the prevalence of UTI due to ESBL producing MDR E. coli in hospitalized patients increased from 0.86% in the period 2003–2006 to 3.11% in the period 2011–2014 (3.6 fold-increase) while this increase in prevalence was more marked, as above, when non-hospitalized patients were analyzed (0.18% in 2003–2006 versus 1.08% in 2011–2014; 6 fold-increase).
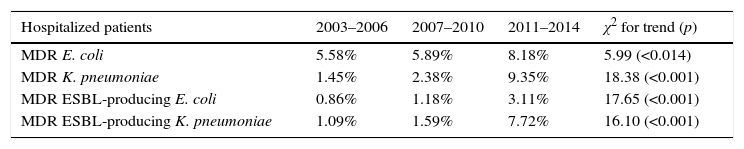
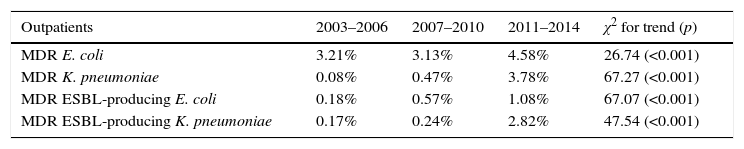
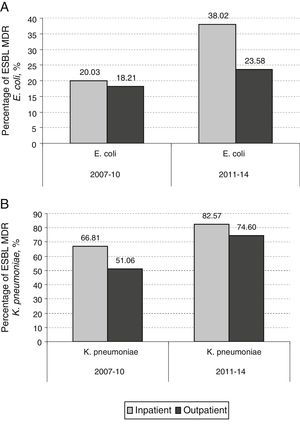
By contrast, the prevalence of UTI due to ESBL producing MDR K. pneumoniae in hospitalized patients increased from 1.09% in the period 2003–2006 to 7.72% in the period 2011–2014 (7.6 fold-increase) while this increase in prevalence was more marked when non-hospitalized patients were analyzed (0.08% in 2003–2006 versus 2.82% in 2011–2014; 35.2 fold-increase). In hospitalized patients, 14 out of 70 (20%) MDR E. coli isolates were MDR ESBL-producing E. coli in 2007–2010. This percentage increased to 38% (30 out of 79) in 2011–2014 (p=0.016). However, the increase observed in non-hospitalized patients between the same periods was not as marked (18.2% in 2007–2010 to 23.6% in 2011–2014) (p=0.085) (Fig. 3A). The contribution of ESBL to the MDR K. pneumoniae isolated in hospitalized patients was 66.8% (4 out of 6) in 2007–2010; this percentage rose to 82.6% (19 out of 23) in 2011–2014 (p=0.77). The increase observed in non-hospitalized patients between the same periods was 51% (4 out of 8) in 2007–2010 to 74.6% (50 out of 67) in 2011–2014 (p=0.143) (Fig. 3B). The results indicate that the contribution of ESBL to the MDR behaviour was more pronounced in K. pneumoniae isolates. Table 2 shows an analysis for linear trend in MDR E. coli and K. pneumoniae in hospitalized patients (Table 2A) and MDR E. coli and K. pneumoniae in non-hospitalized patients (Table 2B) over the period of the study.
Percentage of MDR E. coli (A) and K. pneumoniae (B) urinary isolates from both, hospitalized patients and outpatients over the periods of the study (2003–2006, 2007–2010, and 2011–2013), and chi square test for trend. A p-value<0.05 was considered as statistically significant.
| Hospitalized patients | 2003–2006 | 2007–2010 | 2011–2014 | χ2 for trend (p) |
|---|---|---|---|---|
| MDR E. coli | 5.58% | 5.89% | 8.18% | 5.99 (<0.014) |
| MDR K. pneumoniae | 1.45% | 2.38% | 9.35% | 18.38 (<0.001) |
| MDR ESBL-producing E. coli | 0.86% | 1.18% | 3.11% | 17.65 (<0.001) |
| MDR ESBL-producing K. pneumoniae | 1.09% | 1.59% | 7.72% | 16.10 (<0.001) |
In order to study the role of alternative antibiotic treatments in the MDR isolates, susceptibility to fosfomycin, meropenem and amoxicillin/clavulanate was analyzed. All the MDR E. coli isolates (from both, inpatients and outpatients) were sensitive to fosfomycin during the first 8 years (first and second periods). The proportion of susceptible isolates diminished during the last period (82% from inpatients and 86% from outpatients).
In the same way, all the isolates studied were susceptible to meropenem (except one isolate of MDR K. pneumoniae from a hospitalized patient harbouring a carbapenemase OXA-48 in 2013).
Susceptibility to amoxicillin/clavulanate decreased along the period of the study in both, MDR E. coli and MDR K. pneumoniae. Susceptibility to amoxicillin/clavulanate in MDR E. coli isolates from hospitalized patients decreased from 84.5% in the period 2003–2006 to 46.8% in the period 2011–2014 (1.8-fold decreased). Comparable results were obtained in the same period when outpatient population was analyzed: 86.6% versus 51.7% respectively. Susceptibility to amoxicillin/clavulanate in MDR K. pneumoniae suffered a more drastic change between 2003–2006 and 2011–2014. All the MDR K. pneumoniae isolates from hospitalized patients were resistant to amoxicillin/clavulanate in 2011–2014 respect to the first period (49.6% were susceptible). Susceptibility to amoxicillin/clavulanate in MDR K. pneumoniae isolates from outpatients decreased from 100% in the period 2003–2006 to 16.4% in the period 2011–2014 (6-fold decrease).
DiscussionThe increase of bacterial resistance to antibiotics is a cause of global concern. Infections caused by multiresistant microorganisms frequently fail to respond to a suitable empirical therapy and often times, do not achieve treatment success.13
Few studies have reported on the MDR patterns expressed by Gram negative uropathogens in both, hospitalized and non-hospitalized patients, and the evolution over time of such patterns.7,14–16 Some studies performed at community level have showed a high percentage (about 38%) of MDR in E. coli, the most prevalent bacteria involved in the community-acquired UTI17 while, on the other hand, MDR-Klebsiella species are considered as one of the most common bacteria involved in nosocomial and urinary tract infections.18
The goal of this work was to analyze the evolution of multidrug resistance in both, outpatients and inpatients, to antibiotics commonly used in the treatment of urinary tract infection over a 12-year period. As expected, MDR phenotypes were more usually associated with hospitalization, but the results show that MDR bacteria at the community level are increasing almost at the same rate as in hospitalized patients.
The multiresistance could be associated with the high prescription of these antibiotics groups at community level in Spain.19,20
Quinolone resistance is frequently associated with ESBL-production, and according to the literature, CTX-M represent the most common ESBL produced among bacteria implicated in community-acquired UTI worldwide.21 In our study, a high percentage of strains producing ESBL were detected in MDR isolates from both inpatients and outpatients. The prevalence of infection due to MDR ESBL-producing K. pneumoniae in non-hospitalized patients increased significantly during the last 8 years. On the other hand, exposure to penicillins and/or trimethoprim–sulfamethoxazole has been considered a risk factor for ESBL-producing E. coli isolation.22 All of these results are in accordance with the results obtained in our study suggesting that resistance to quinolones and trimethoprim–sulphamethoxazole are associated with ESBL production. By contrast, this phenomenon was less marked in hospitalized patients.
It is important to take into account that alternative therapies such as fosfomycin represent a promising treatment option against MDR urinary isolates. Fosfomycin resistance is usually rare in areas with limited use but it is increasing in countries with higher usage.23 Our results show that all the MDR E. coli isolates were sensitive to fosfomycin during the first 8 years, but resistant isolates have emerged during the last years. By contrast all the MDR isolates were susceptible to carbapenems due to the absence of carbapenemases.
The emergence and spread of different clones have to be taken into account in order to explain the increase in MDR bacteria. Numerous articles have reported that the well-known high-risk E. coli ST131 and K. pneumoniae ST258 clones have been responsible for the sudden increase in multidrug resistance.24–27 ST131 is responsible of UTI, frequently fluoroquinolone resistant and can be linked with the production of CTX-M-15,28 while ST258 is also known to cause UTI, respiratory tract infections, and blood stream infections and is associated with carbapenemase production, most often KPC-2 and KPC-3.29 Unfortunately, we do not have information about molecular characterization of the strains isolated in our study.
It is also important to take into consideration that mobile elements (plasmids, transposons, integrons, etc.) play an important role in the transfer of other resistance genes and thus allowing a co-selection phenomenon (see for a review30).
The present study has some limitations; it was conducted in an area (Getafe), which represents a small portion of the total population of Spain and, in this case, the results cannot be extrapolated to other settings because specific epidemiology of different areas have to be taken into consideration. Further studies would be necessary to address the problem at national and global level. Despite the above limitations, some strengths are also remarkable and include data of a large sample size of urinary strains isolated over a 12 year period that demonstrates that MDR is an increasing phenomenon of particular importance over the last few years.
FundingNo specific funding has been received.
Conflict of interestNone to declare.