Catheter-related bloodstream infections (CRBSI) constitute an important cause of hospital-acquired infection associated with morbidity, mortality, and cost. The aim of these guidelines is to provide updated recommendations for the diagnosis and management of CRBSI in adults. Prevention of CRBSI is excluded. Experts in the field were designated by the two participating Societies (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica and the Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias). Short-term peripheral venous catheters, non-tunneled and long-term central venous catheters, tunneled catheters and hemodialysis catheters are covered by these guidelines. The panel identified 39 key topics that were formulated in accordance with the PICO format. The strength of the recommendations and quality of the evidence were graded in accordance with ESCMID guidelines. Recommendations are made for the diagnosis of CRBSI with and without catheter removal and of tunnel infection. The document establishes the clinical situations in which a conservative diagnosis of CRBSI (diagnosis without catheter removal) is feasible. Recommendations are also made regarding empirical therapy, pathogen-specific treatment (coagulase-negative staphylococci, Sthaphylococcus aureus, Enterococcus spp, Gram-negative bacilli, and Candida spp), antibiotic lock therapy, diagnosis and management of suppurative thrombophlebitis and local complications.
La bacteriemia relacionada con catèc)teres (BRC) constituye una causa importante de infección hospitalaria y se asocia con elevada morbilidad, mortalidad y costo. El objetivo de esta guía de práctica clínica es proporcionar recomendaciones actualizadas para el diagnóstico y el tratamiento de la BRC en pacientes adultos. De este documento se excluye la prevención de la BRC. Expertos en la materia fueron designados por las 2 sociedades participantes (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica y Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias). Los catèc)teres venosos perifèc)ricos a corto plazo, los catèc)teres venosos centrales no tunelizados y de largo plazo, los catèc)teres tunelizados y los catèc)teres de hemodiálisis están incluidos por estas guías. El panel identificó 39 temas claves que fueron formulados de acuerdo con el formato PICO. La fuerza de las recomendaciones y la calidad de la evidencia se clasificaron de acuerdo con las directrices de la ESCMID. Se hacen recomendaciones para el diagnóstico de BRC con y sin extracción de catèc)ter y de la infección en túnel. El documento establece las situaciones clínicas en las que es factible un diagnóstico conservador de CRBSI (diagnóstico sin retirada de catèc)ter). Tambièc)n se hacen recomendaciones con respecto a la terapia empírica, el tratamiento específico según el patógeno identificado (estafilococos coagulasa negativos, Staphylococcus aureus, Enterococcus spp, bacilos gramnegativos y Candida spp), la terapia con sellado del catèc)ter, el diagnóstico, así como el tratamiento de la tromboflebitis supurativa y las complicaciones locales.
Intravascular devices have become an essential component of modern medicine for the administration of intravenous fluids, medication, blood products and parenteral nutrition and for monitoring hemodynamic status and providing hemodialysis. According to national data supplied by the study of the prevalence of nosocomial infections in Spain (EPINE), it is estimated that about 70% of patients admitted to Spanish hospitals will wear one of these devices at some point during their stay.1 Local or systemic infections represent one of the main associated complications.2 The incidence of catheter-related infections varies considerably depending on the type and intended use, the insertion site, the experience and training of the individual who places the catheter, the frequency with which the catheter is accessed, duration of catheter placement, the characteristics of the patient, and the use of proven prevention strategies. Catheter-related bloodstream infections (CRBSIs) are among the most frequent infections acquired in hospital. Current estimates are that between 15 and 30% of all nosocomial bacteremias are catheter-related.3 CRBSIs have significant associated morbidity, incur increased hospital costs,4 estimated at approximately 18,000 euros per episode, and length of stay.5 Attributable mortality ranges between 12 and 25%.6 In recent years, there has been a remarkable increase in our knowledge of the epidemiology of CRBSI and of the most appropriate methodologies for diagnosis, management and prevention. The vast amount of information accumulated and the inherent complexity of this type of infection make it necessary to sort and analyze the available information. At the same time, there are few current guidelines available on this topic. The last Spanish catheter-related infections guidelines were published in 2004.7 The aim of this new guide is to update recommendations for the diagnosis and management of catheter-related bloodstream infections. This document targets only microbiological diagnosis and antimicrobial therapy; other aspects of infection management and prevention are therefore excluded. Only adult patients with these infections are covered.
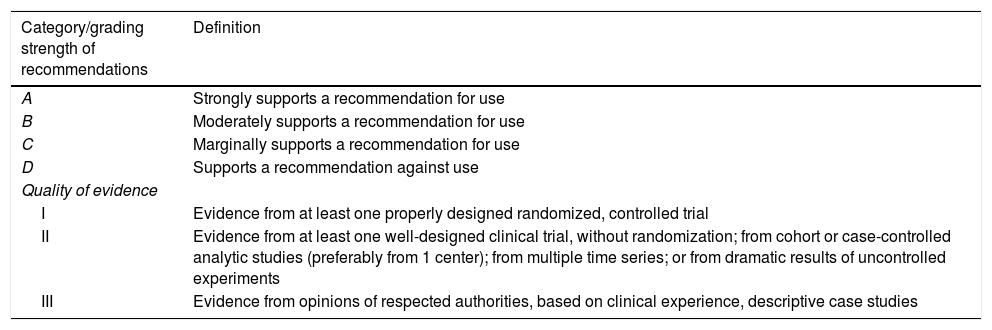
MethodsThe two participating Societies (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica and the Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias) nominated three coordinators for this project (FC, JGM and JLdP: a microbiologist, an intensivist, and an infectious disease physician). This coordinating group selected the rest of the members of the panel, including microbiologists, intensivists, and infectious disease physicians. The Scientific Committees of both Societies approved their proposal. The present Statement was written following the SEIMC guidelines for consensus statements (www.seimc.org) as well as the recommendations of the AGREE Collaboration (www.agreecollaboration.org) for evaluating the methodological quality of clinical practice guidelines. The strength of the recommendations and quality of the evidence were graded in accordance with ESCMID guidelines (Table 1).
Strength of recommendation and quality of evidence.
| Category/grading strength of recommendations | Definition |
|---|---|
| A | Strongly supports a recommendation for use |
| B | Moderately supports a recommendation for use |
| C | Marginally supports a recommendation for use |
| D | Supports a recommendation against use |
| Quality of evidence | |
| I | Evidence from at least one properly designed randomized, controlled trial |
| II | Evidence from at least one well-designed clinical trial, without randomization; from cohort or case-controlled analytic studies (preferably from 1 center); from multiple time series; or from dramatic results of uncontrolled experiments |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive case studies |
The coordinating group identified 39 key topics that were formulated in accordance with the PICO format defining the population, intervention, comparator, and outcome of interest. These key questions were approved by the Scientific Committees of both Societies and then distributed to the different members of the panel (2 or 3 questions each) for further development. The coordinating group wrote the first draft based on the sections submitted by each participant, which was then sent to the panel for critical review. Before its final approval, the document was published on the intranet of both Societies and left open to suggestions and comments from members. All authors and coordinators of the Statement have agreed the contents of the document and the final recommendations.
General aspectsWhen should catheter-related bloodstream infection be suspected?
Recommendations:
- •
CRBSI should be suspected in patients with intravenous catheters and fever, chills or other signs of sepsis, even in the absence of local signs of infection, and especially if no alternative source is identified (A-III).
- •
Clinical suspicion of CRBSI should also increase in patients with intravenous catheters who have metastatic infections caused by hematogenous spread of microorganisms (i.e. septic emboli) (A-III).
- •
Persistent or recurrent bacteremia caused by microorganisms that tend to colonize or infect the skin in patients with intravenous catheters should lead to suspicion of CRBSI (A-III).
How is a complicated catheter-related bloodstream infection defined?
Recommendations:
- •
Patients with CRBSI with endocarditis, suppurative thrombophlebitis, septic metastasis, extraluminal infections, septic shock, non-resolving CRBSI, and immunocompromised patients should be categorized as complicated CRBSI (A-III).
How should blood cultures be taken?
Recommendation:
- •
Obtain blood cultures using an aseptic technique before the initiation of antimicrobial therapy (A-I)
- •
Skin preparation for obtaining blood samples drawn percutaneously should be performed with proper techniques, including the time to perform the procedure and leaving adequate time for the disinfectant to take effect (A-I). Alcohol-containing products are associated with low rates of contamination. Alcohol-chlorhexidine solutions reduce blood culture contamination more efficiently than aqueous povidone-iodine (A-I).
- •
In patients with suspected CRBSI, two pairs of blood cultures should be drawn, one from a peripheral vein and the other from the catheter (A-I).
- •
For multiple-lumen venous catheters, samples for blood culture should be obtained from all lumens (A-II).
How should conventional blood cultures be interpreted?
Recommendation:
- •
For diagnosis of CRBSI, positivity of blood cultures obtained through the catheter ≥120min before those from a peripheral vein with the same microorganism is highly suggestive. An optimal DTP cut-off for the diagnosis of catheter-related candidemia has not been established. (A-II).
- •
The interpretation of DTP should consider adherence to the procedural technique used and the type of microorganism (A-II).
- •
Rapid microbial identification by MALDI-TOF MS from a positive blood culture significantly reduces time to identification of microorganisms and has clinical impact on the management of patients with suspected bloodstream infection (A-II).
How should quantitative blood cultures be taken and interpreted?
Recommendation:
- •
A quantitative blood culture with a colony count 3 times greater in a sample drawn through a catheter than from the peripheral vein supports a diagnosis of CRBSI (A-II). This method is less practicable for routine use.
What particular aspects should be considered for the diagnosis of CRBSI in patients on hemodialysis?
Recommendations:
- •
Whenever possible, paired blood samples from the CVC and a peripheral vein should be obtained for a CRBSI diagnosis in hemodialysis patients (A-II).
- •
Peripheral blood samples should be obtained from veins that are not intended for future creation of dialysis fistulae or grafts. The veins of the hand for outpatients and hand or femoral veins for hospital inpatients should be used to obtain peripheral blood cultures (A-III).
- •
If a blood sample cannot be drawn from a peripheral vein, two separate samples should be drawn, 10•15min apart, through the CVC or the dialysis circuit connected to the catheter (B-II).
What is the present value of molecular techniques for the diagnosis of CRBSI?
Recommendations:
- •
At the present time, there is not enough information to recommend implementing these techniques in clinical practice for CRBSI diagnosis (C-II).
When should a catheter tip be sent for culture?
Recommendations:
- •
Catheter cultures should only be obtained when catheter-related bloodstream infection is suspected (A II).
How should a catheter be sent and processed in the Microbiology Laboratory?
Recommendations:
- •
The most reliable diagnostic methodologies are the semiquantitative (roll plate) or quantitative (vortex or sonication methods) catheter culture techniques (A-II).
- •
Qualitative cultures (culture of the catheter tip by broth immersion) are unreliable for distinguishing between contamination and infection and are not therefore suitable for the diagnosis of CRBSI (A-II).
How should the results of catheter cultures be interpreted?
Recommendations:
- •
The presence of 15 CFU per plate or more by semiquantitative culture (roll-plate) is indicative of significant catheter colonization (A-II).
- •
For quantitative culture methods based on vortexing or flushing the internal surface, a count of 103CFU/segment or more reflects significant catheter colonization (A-II).
- •
For quantitative culture methods based on sonication, counts above 102 CFU/segment indicate significant catheter colonization (A-II).
How should a subcutaneous reservoir be processed?
Recommendations:
- •
Venous access devices removed for suspected CRBSI should be sent to the microbiology laboratory. Routine processing should include a combination of cultures from different parts of the VAD, including a culture after septum sonication and semiquantitative catheter tip cultures (B-II).
What other conservative techniques may be used for diagnosis of CRBSI?
Recommendation:
- •
Endoluminal brushing of the internal surface of the catheter may be useful for diagnosis of CRBSI. However, the procedure is impractical and major side-effects have been reported (C-III).
- •
Semiquantitative cultures of skin around the catheter insertion site and catheter hubs with ≥15cfu may be indicative for CRBSI. These procedures must be combined with peripheral blood culture (B-II).
- •
Gram stain • acridine orange leukocyte cytospin (AOLC) of catheter blood may be used as a rapid method for diagnosis of CRBSI. The presence of any microorganisms in a minimum of 100 high-powered fields may be indicative of CRBSI (B-II).
What is the value of molecular techniques for the diagnosis of CRBSI after catheter removal?
Recommendation:
- •
16S rRNA PCR could be performed with septum sonication fluid to rule out or confirm VAD-RBSI in patients undergoing antibiotic therapy (C-III).
What samples should be taken and how should they be interpreted when an insertion site infection is suspected?
Recommendations:
- •
When catheter infection is suspected and there is exudate at the catheter insertion site, it should be sent for Gram staining and culture. Blood cultures should also be drawn (A-III).
- •
In patients with suspected catheter-related infection but negative superficial cultures (growth of <15CFU from both the insertion site and catheter hub cultures), the possibility of infection can reasonably be ruled out (B-II).
When can a catheter be retained until blood cultures are available?
Recommendation:
- •
Immediate removal of the CVC is not routinely recommended when CRBSI is suspected in patients who are hemodynamically stable, without autoimmune diseases or immunosuppressive therapy, intravascular foreign bodies or organ transplants, no suppuration at the insertion site or bacteremia/fungemia (A-I).
When is it safe to perform a catheter exchange over a guidewire?
Recommendations:
- •
Routine replacement of a CVC by guidewire exchange is not recommended because this strategy is associated with a higher risk of infectious complications. (B-II)
- •
Guidewire exchange of a CVC is contraindicated in patients with documented catheter infections. (A-II)
- •
Guidewire exchange should be restricted to patients with very difficult venous access (i.e. extensive burns, morbid obesity, or severe coagulopathy) and without documented catheter infection (B-II). In this case, a meticulous aseptic technique and a culture of the catheter tip are mandatory. (A-III)
- •
If the catheter tip culture is positive, the new line, inserted over a guidewire, should be re-placed via a new direct venipuncture. (C-III)
What should be done if the catheter tip culture is positive but the blood cultures are negative?
Recommendations:
- •
Antibiotic treatment (i.e. 5•7 days) should be given to patients with catheter tip cultures positive for S. aureus and negative blood cultures if the patient shows systemic signs of infection or signs of local infection (B-II).
- •
In non-neutropenic patients or those without valvular heart disease, the presence of a catheter tip culture positive for Candida spp. and negative or unavailable blood cultures should be assessed on an individual basis before starting systematic antifungal treatment. Antifungal treatment should not be prescribed for patients without systemic signs of infection (B-II).
- •
No clear recommendations can be given for catheters colonized with other microorganisms (C-III).
What is the empirical antimicrobial therapy for CRBSI?
Recommendations:
- •
If CRBSI is suspected, antimicrobial therapy should be started as soon as possible with a bactericidal agent active against S. aureus and CoNS, especially if associated with sepsis or septic shock (B-II).
- •
Vancomycin is recommended for empirical therapy in patients with suspected CRBSI (B-II). Teicoplanin is not recommended as empirical therapy, given the existence of coagulase-negative staphylococci with reduced susceptibility to teicoplanin (C-III).
- •
Daptomycin can be administered for cases of CRBSI with septic shock (C-III), acute kidney injury (B-III), to patients with recent exposure to vancomycin (>1 week in the past 3 months) (C-III) or if the local prevalence of S. aureus isolates with vancomycin MIC≥2.0α/4g/ml is high (C-III). The local prevalence of S. aureus isolates with vancomycin MIC≥1.5α/4g/ml supporting routine empirical use of daptomycin remains undefined.
- •
Linezolid should only be used in patients with contraindications for the previous agents (B-II).
When should empirical coverage of Gram-negative bacilli or fungi be added?
Recommendations:
- •
Patients with suspected CRBSI should receive empirical antibiotic therapy (in addition to coverage for Gram-positive pathogens) to cover Gram-negative bacilli under any of the following circumstances: hemodynamic instability (septic shock), neutropenia or hematologic malignancy, solid organ or bone marrow transplant, femoral catheter in place, a high index of colonization with Gram-negative bacilli or prolonged ICU admission (C-III).
- •
Antimicrobial therapy should be adapted to local epidemiology and must include an antipseudomonal agent (piperacillin-tazobactam, carbapenems, a fourth-generation cephalosporin, aztreonam, quinolones or aminoglycosides) (A-II). Aztreonam and cephalosporins should be avoided in patients with colonization or at risk for extended-spectrum β-lactamase infections (A-I).
- •
The need for empirical antifungal therapy in a patient with suspected catheter-related candidemia should be evaluated along with the possibility of catheter removal (A-III).
- •
Empirical therapy for suspected catheter-related candidemia should be considered in patients who are hemodynamically unstable with one or more of the following conditions: total parenteral nutrition, prolonged use of broad-spectrum antibiotics, malignancy, femoral catheterization, colonization due to Candida species at multiple sites or intense previous anti-anaerobic therapy (C-III).
- •
The use of biomarkers (such as 1,3-beta-D-glucan) may be useful when considering initiation of empirical treatment (B-III).
What particular aspects should be considered in the empirical treatment of CRBSI in patients on hemodialysis?
Recommendation:
- •
Conservative management of CRBSI should be attempted with hemodialysis patients. Combining systemic and local intracatheter antibiotics is associated with improved results compared to systemic antibiotics alone (A-I).
- •
In patients with a tunneled hemodialysis catheter, guidewire exchange is an alternative, especially when catheter removal is not feasible (C-III).
What is the recommended directed therapy and optimal duration of treatment for CRBSI due toStaphylococcus aureus?
Recommendations:
- •
The treatment of choice for an episode of MSSA CRBSI is cloxacillin or cefazoline (B-I).
- •
Patients allergic to beta-lactams should be treated with daptomycin (A-I) or a glycopeptide (B-II).
- •
The best antimicrobial treatment for episodes caused by MSSA strains with reduced susceptibility to vancomycin (MIC≥1.5mg/L measured by E-test) has not been elucidated. This panel suggests using a combination of cloxacillin and daptomycin when blood cultures remain positive and/or there is no obvious clinical improvement after catheter removal (C-III).
- •
Vancomycin is the treatment of choice for CRBSI caused by MRSA (B-II). Teicoplanin may be a valid alternative, especially in cases of serious side effects associated with the use of vancomycin. (C-III)
- •
Alternatively, patients may be treated with daptomycin, specifically if the MIC measured by E-test is ≥1.5mg/L (A-I).
- •
Linezolid should only be used in patients when the previous agents are contraindicated (C-III).
- •
In both MSSA and MRSA CRBSI, blood cultures should be obtained after 72h of antibiotic therapy (C-III).
What is the recommended directed therapy and optimal duration of treatment for CRBSI due to coagulase-negative Staphylococcus (CoNS)?
Recommendations:
- •
Cloxacillin or cefazolin are the treatments of choice for episodes of CRBSI caused by CoNS susceptible to methicillin (B-I).
- •
For CoNS resistant to methicillin, a glycopeptide is the treatment of choice for directed therapy (B-II). Teicoplanin is recommended in the case of serious side effects associated with vancomycin. (C-III).
- •
The optimal trough concentration of vancomycin for the treatment of CoNS CRBSI is an unresolved issue and this panel cannot issue a specific recommendation (C-III).
- •
S. lugdunensis CRBSI should be managed as for S aureus CRBSI (C-III).
What is the recommended directed therapy and its optimal duration for CRBSI due toEnterococcusspp.?
Recommendations:
- •
Enterococcal CRBSI should be treated with catheter withdrawal and one active antimicrobial (A-III).
- •
Ampicillin is the drug of choice for susceptible isolates (A-II). Vancomycin should be reserved for isolates resistant to ampicillin or cases of beta-lactam allergy. For vancomycin-resistant isolates or severe adverse effects, linezolid is preferred to daptomycin (B-III).
- •
There is no evidence that combination therapy is necessary if IE has been properly ruled out (A-III).
- •
Despite data suggesting that duration of treatment may be shorter, the standard 7•14 day regimen continues to be recommended (A-III).
What is the recommended directed therapy and its optimal duration for CRBSI due to Gram-negative bacilli?
Recommendations:
- •
Directed therapy for GN-CRBSI should be chosen on the basis of the susceptibility results (C-III).
- •
The appropriate length of antimicrobial therapy has not been elucidated, although it is recommended to continue therapy for at least 7 days (C-II).
What is the recommended directed therapy and its optimal duration for CRBSI due toCandidaspp.?
Recommendations:
- •
In patients with Candida spp CRBSI, this panel advocates de-escalation from an echinocandin or a lipid formulation of amphotericin B to fluconazole for susceptible isolates in clinically stable patients who have undergone catheter removal (B-II).
- •
The recommended duration of therapy for candidemia without obvious metastatic complications is two weeks after the first set of negative blood cultures (B-III).
- •
In candidemia, all intravascular catheters should be removed if at all feasible (B-II), particularly in patients with septic shock and Candida CRBSI is suspected (B-III).
- •
If a catheter that is the source of a Candida bloodstream infection cannot be removed for any reason and remains in place, an antifungal agent with high activity against biofilms should be used (i.e. an echinocandin or liposomal amphotericin B) (A-II).
What is the recommended directed therapy and its optimal duration for CRBSI due to nontuberculous mycobacteria (NTM)?
Recommendations:
- •
The treatment for CRBSI caused by NTM involves removal of the infected catheter (B-II) followed by combination antimicrobial treatment appropriate for the species involved (B-III).
- •
The duration of treatment for NTM CRBSI should be 6•12 weeks to prevent recurrence of infection and the development of septic metastases (B-III).
Should antimicrobials for CRSBI be administered intravenously for the entire course of treatment?
Recommendations:
- •
Sequential oral therapy can be considered in clinically stable patients without metastatic complications and with negative blood cultures after onset of treatment and removal of the intravenous line, if a therapeutic option with high oral bioavailability is available (A-II).
- •
In uncomplicated CRBSI caused by fluoroquinolone-susceptible staphylococci, initial intravenous antibiotic treatment may be switched to high-dose oral fluoroquinolones plus rifampicin in order to complete the course of antibiotic therapy if the patient is clinically stable and clearance of bacteremia is documented. Linezolid could be an option if the microorganism involved is fluorquinolone-resistant (A-II).
- •
In uncomplicated CRBSI caused by fluoroquinolone-susceptible Gram-negative bacilli, initial intravenous antibiotic treatment may be switched to high-dose oral fluoroquinolones in order to complete the course of antibiotic therapy if the patient is clinically stable and clearance of bacteremia is documented (A-II).
- •
A step-down from an echinocandin or lipid formulation of amphotericin B to oral fluconazole is safe and effective (C-III).
When is conservative management with antibiotic lock therapy recommended?
Recommendation:
- •
Conservative treatment should not be prescribed for patients with metastatic or local septic complications (A-II).
- •
The use of lock therapy added to systemic antimicrobial agents is systematically recommended for infected catheters that fulfill the criteria for catheter retention: the patient is stable and the microorganism involved is considered to be of low virulence (i.e. CoNS) (A-I).
- •
In stable patients without local or systemic complications, conservative treatment may also be attempted for enterococci, corynebacterium (except Corynebacterium jeikeium) and Gram-negatives (consultation with an ID expert is suggested in such cases) (C-III).
- •
The use of an antibiotic lock does not preclude the need for systemic antimicrobial therapy (A-I).
What antibiotics and concentrations of antibiotic lock solutions are recommended?
Recommendation:
- •
The most frequently used antibiotics for conservative treatment of CRBSI using ALT are vancomycin 2000mg/L, teicoplanin 10,000mg/L, daptomycin 5000mg/L, ciprofloxacin 2000mg/L, and amikacin 2000mg/L (B-I).
How should antibiotic lock therapy be performed?
Recommendation:
- •
An ALT solution should be prepared under sterile conditions. It should be infused after removing the previous dose and the exact volume of the catheter lumen should be infused. The recommended duration of ALT is 10•14 days. The ALT solution must remain in the catheter lumen for a minimum of 12h a day and should be replaced every 24•72h (B-I).
What non-antibiotic substances could be used for lock therapy?
Recommendations:
- •
70% ethanol and taurolidine locks can also be used for the conservative treatment of CRBSI. There is no evidence to advocate for their routine use (B-I).
What are the criteria for failure of conservative management?
Recommendation:
- •
Any clinical condition or catheter dysfunction prompting catheter removal should be considered failure of conservative management (A-I).
How should insertion site infection be managed?
Recommendations:
- •
For peripheral venous catheters, catheter removal is mandatory if there is local pain, induration, erythema or exudate (A-I).
- •
For non-tunneled CVCs, the presence of erythema or purulence at the catheter insertion site requires immediate catheter removal (B-II).
- •
For uncomplicated exit site infections with long-term catheters, a conservative approach with topical antimicrobial agents should first be attempted. In cases of topical treatment failure, systemic antibiotics should be administered (B-III).
- •
Persistence of clinical signs of infection beyond 72h of conservative management requires removal of the catheter (B-II).
How should tunnelitis be managed?
Recommendations:
- •
Patients with tunnel infection not associated with a hemodialysis catheter require catheter removal, incision and drainage, if indicated, and 7•10 days of systemic antimicrobial therapy in the absence of concomitant bacteremia or candidemia (A-II).
- •
For tunnelitis without fever in hemodialysis catheters, systemic antibiotic therapy may be attempted first (A-II). In tunnel infection with fever, catheter removal is the first therapeutic option together with systemic antimicrobial therapy (A-II).
- •
In tunnelitis, conservative management is associated with higher failure rates (B-II).
How should a local infection associated with a port reservoir be managed?
Recommendations:
- •
In the presence of signs of local inflammation at a port reservoir, the port must be removed, the affected tissue drained and systemic antibiotic therapy started (A-II).
- •
If a conservative strategy is the only option, a combination of systemic antibiotics and antibiotic lock therapy should be prescribed, bearing in mind that this approach is associated with a high failure rate (B-II).
In which patients and when should a follow-up blood culture be taken?
Recommendations:
- •
Follow-up blood cultures should be taken from all patients with S. aureus or Candida spp CRBSI (A-II).
- •
In patients with S. aureus CRBSI, we recommend that follow-up blood cultures should be obtained every 72h until the first negative result (A-II).
- •
Control blood cultures in CRBSI due to Candida spp should be obtained every 48h until the first negative blood culture (A-II).
- •
For other causative microorganisms of CRBSI and if catheter salvage is attempted, follow-up blood cultures should be obtained 72h after starting appropriate antibiotic therapy. If persistent bacteremia is documented, catheter removal is required (B-II).
- •
It is not necessary to routinely perform follow-up blood cultures in patients with CRBSI due to microorganisms other than S. aureus or Candida spp if the catheter has been withdrawn (A-II).
When should echocardiography be performed?
Recommendations:
- •
TEE should be performed in the vast majority of patients with Staphylococcus aureus bacteremia. TEE is not necessary or can be delayed in patients without the following risk factors: prolonged bacteremia, hemodialysis, metastatic foci of infection, IVDA, implantable CVC, intracardiac device, prosthetic valve, previous IE or cardiac structural abnormality (A-II).
- •
The need for TEE in episodes of CRBSI caused by other pathogens should be individualized. This panel considers that IE should be ruled out in all patients with persistent bacteremia (or fungemia) (C-III). Enterococcus spp and Candida spp pathogens are associated with a high risk of developing endocarditis.
What is the diagnosis and management for w?
Recommendation:
- •
Suppurative thrombophlebitis should be ruled out in all episodes of CRBSI with persistent bacteremia (A-II).
- •
Confirmed diagnosis, mainly by ultrasonography, should be followed by catheter withdrawal, prolonged antibiotic treatment and an individualized assessment of the need for anticoagulation (A-II).
When can a new catheter be inserted?
Recommendation:
- •
Although there is a clear lack of scientific evidence, it seems advisable to wait, if feasible, before placing a new catheter after an episode of CRBSI. The waiting period should be determined by the resolution of signs and symptoms. If a patient urgently needs vascular access, a catheter should be inserted without delay (C-III).
- •
Insertion of a new catheter after a diagnosis of CRBSI is always possible if the patient's clinical condition dictates the need for a new vascular access (A-III).
Jaime Esteban has participated in counseling and lectures at meetings sponsored by Laboratorios Leti SL in the last year, as well as received grants for research and teaching from laboratories Pfizer, Sysmex, Angelini and bioMèc)rieux.
Paula Ramirez has participated in counseling and lectures at meetings sponsored by Pfizer, MSD, Astellas, Gilead and Otsuka in the last year, as well as received grants for research and teaching at Otsuka laboratories.
Jose Luis del Pozo has lectured at meetings sponsored by Pfizer, MSD and Angelini Laboratories.
Miguel Salavert has participated in counseling and lectures at meetings sponsored by MSD, Pfizer, Gilead, Astellas Ph and Angelini in the last year, as well as received grants for research and teaching from Janssen and ViiV laboratories.
Josèc) Ramón Paño has participated in counseling and lectures at meetings sponsored by Janssen, Gilead and MSD in the last year.
Josèc) Garnacho-Montero has participated in conferences sponsored by MSD and Astellas.
Emilio Bouza has participated in advisory services and lectures sponsored by MSD, Pfizer and Astellas in the last year.
The rest of the authors declare that they have no conflicts of interest.
The authors thank Dr. Antonio Gutièc)rrez-Pizarraya for his commentaries and technical support for the elaboration of this document.
The consensus statement is available at: https://www.seimc.org/contenidos/documentoscientificos/guiasclinicas/seimc-guiasclinicas-2017-Catheter-Related_Bloodstream_Infection.pdf and as additional material in the journal official website.
The complete consensus statement has also been published in: Medicina Intensiva. 2017. http://dx.doi.org/10.1016/10.1016/j.medin.2017.09.012