Antiretroviral therapy (ART) is recommended for all patients infected by HIV-1. The objective of ART is to achieve an undetectable plasma viral load (PVL). Initial ART should be based on a combination of 3 drugs, including 2 nucleoside reverse transcriptase inhibitors (tenofovir in either of its two formulations plus emtricitabine or abacavir plus lamivudine) and another drug from a different family. Four of the recommended regimens, all of which have an integrase inhibitor as the third drug (dolutegravir, elvitegravir boosted with cobicistat or raltegravir), are considered preferential, whereas a further 3 regimens (based on elvitegravir/cobicistat, rilpivirine, or darunavir boosted with cobicistat or ritonavir) are considered alternatives. We present the reasons and criteria for switching ART in patients with an undetectable PVL and in those who present virological failure, in which case salvage ART should include 3 (or at least 2) drugs that are fully active against HIV. We also update the criteria for ART in specific situations (acute infection, HIV-2 infection, pregnancy) and comorbidities (tuberculosis or other opportunistic infections, kidney disease, liver disease and cancer).
Se recomienda tratamiento antirretroviral (TAR) a todas las personas con infección por VIH-1. El objetivo del TAR es lograr una carga viral plasmática (CVP) indetectable. El TAR inicial debe ser una combinación de 3 fármacos, que incluya 2 inhibidores de la transcriptasa inversa análogos de nucleósidos (tenofovir en cualquiera de sus dos formas más emtricitabina o abacavir más lamivudina) y otro de distinta familia. Cuatro de las pautas recomendadas, todas las cuales tienen un inhibidor de la integrasa como tercer fármaco (dolutegravir, elvitegravir potenciado con cobicistat o raltegravir), se consideran preferentes, mientras que otras tres, (basadas en elvitegravir/cobicistat, rilpivirina o darunavir potenciado con cobicistat o ritonavir), como alternativas. Se exponen las causas y criterios para cambiar el TAR en los pacientes con carga viral plasmática indetectable así como en los que presentan fracaso virológico, en cuyo caso el TAR de rescate debe incluir 3 (o al menos 2) fármacos plenamente activos frente al VIH. Se actualizan los criterios específicos del TAR en situaciones especiales (infección aguda, infección por VIH-2, embarazo) o comorbilidades (tuberculosis u otras enfermedades oportunistas, enfermedad renal, hepatopatías y neoplasias).
The complexity and speed of changes of antiretroviral therapy (ART) requires frequent updating of specific guidelines. For the last 18 years, GESIDA and the National AIDS Plan have jointly edited a consensus document on ART in adults.1 The objective of this consensus document, which updates previous recommendations,2 is to provide health professionals who treat HIV-infected adults with up-to-date knowledge on ART and a series of recommendations based on scientific evidence that can act as guidelines in therapeutic decision making.
Clinical and laboratory evaluation as a guide for ARTClinical evaluationRecommendations
- •
A clinical history should be taken for all HIV-infected patients. The history should include an evaluation of the patient's drug therapy, comorbid conditions, and risk of STI. The patient should also undergo a thorough physical examination, which should be repeated once a year (A-II).
- •
In all newly diagnosed cases, all previous sexual contacts should be evaluated after agreement with the index case and with confidentiality guaranteed (B-III).
Recommendations
- •
Serology testing for HIV should be performed in all cases where HIV infection has not been confirmed and the plasma viral load (PVL) is undetectable (A-I).
- •
The initial laboratory workup should include a complete blood count, general biochemistry, and serology testing (Toxoplasma, cytomegalovirus, syphilis, HAV, HBV, and HCV). Specific tests, including viral load, CD4+ T-lymphocyte count, primary resistance to HIV and HLA-B*5701, should also be performed (A-II).
Recommendations
- •
The absolute number and percentage of CD4+ T lymphocytes should be determined before initiating ART. Once therapy has started, these determinations should be made periodically every 3–6 months to monitor the immune response (A-I).
- •
Determinations can be at longer intervals, at the physician's discretion, in stable patients with suppressed plasma viral load (PVL) and CD4+ T-lymphocyte counts >300cells/μL (C-II).
Recommendations
- •
PVL should be determined before initiation of ART and regularly during treatment (A-II).
- •
PVL is the main parameter for evaluating the virological efficacy of ART and for defining virological failure (A-I).
- •
The objectives of virological suppression (VL <50copies/mL) should be met both in ART-naïve patients and in those who have experienced previous therapeutic failure (A-II).
- •
PVL should be determined using a technique with a quantification limit of at least 50copies/mL. The same technique should always be used (A-II).
- •
If decisions on therapy are to be taken based on PVL, they should be confirmed with a second determination (A-II).
Recommendations
- •
Determination of the plasma concentration of antiretroviral drugs is not recommended for habitual monitoring of HIV-infected patients (A-II).
- •
Determination of the plasma concentration of antiretroviral drugs may be indicated in specific clinical situations (e.g., risk of pharmacological interactions, organ transplantation, extreme underweight or overweight, pregnancy, and renal or hepatic insufficiency) and to confirm suspected poor adherence to therapy (B-III).
Recommendations
- •
Genotyping of reverse transcriptase and protease to detect HIV resistance mutations should be performed in all patients at diagnosis of infection and before initiating ART if this is deferred (A-II).
- •
The result of the genotyping study should be known before starting ART with non-nucleoside reverse transcriptase inhibitors (NNRTI) (A-II).
- •
Assessment of baseline integrase resistance mutations is only recommended when there is a high suspicion of transmission of resistance to integrase strand transfer inhibitors (INSTI) (C-III).
- •
Resistance should be studied by genotyping in all patients in whom virological failure has been confirmed. The study should include integrase resistance mutations if the patient's regimen includes an INSTI (A-I).
Recommendations
- •
HLA-B*5701 should be determined in all patients before initiating an ART regimen containing ABC (A-I).
- •
ABC should not be prescribed if the result of the HLA-B*5701 determination is positive (A-I).
Recommendations
- •
HIV-1 tropism should be determined before prescribing a CCR5 receptor antagonist (A-I).
- •
HIV-1 tropism should be determined if a regimen containing a CCR5 receptor antagonist fails (A-I).
Recommendations
- •
ART should be initiated in all HIV-infected patients.
- •
Initiation of ART should always be evaluated on an individual basis. Both CD4+ T-lymphocyte count and PVL should be determined before initiating ART. Furthermore, the patient should be briefed on the various options available, and the therapeutic regimen should be adapted to lifestyle, comorbid conditions, and possible drug interactions. The risk of poor adherence should also be assessed (A-III).
Recommendation
- •
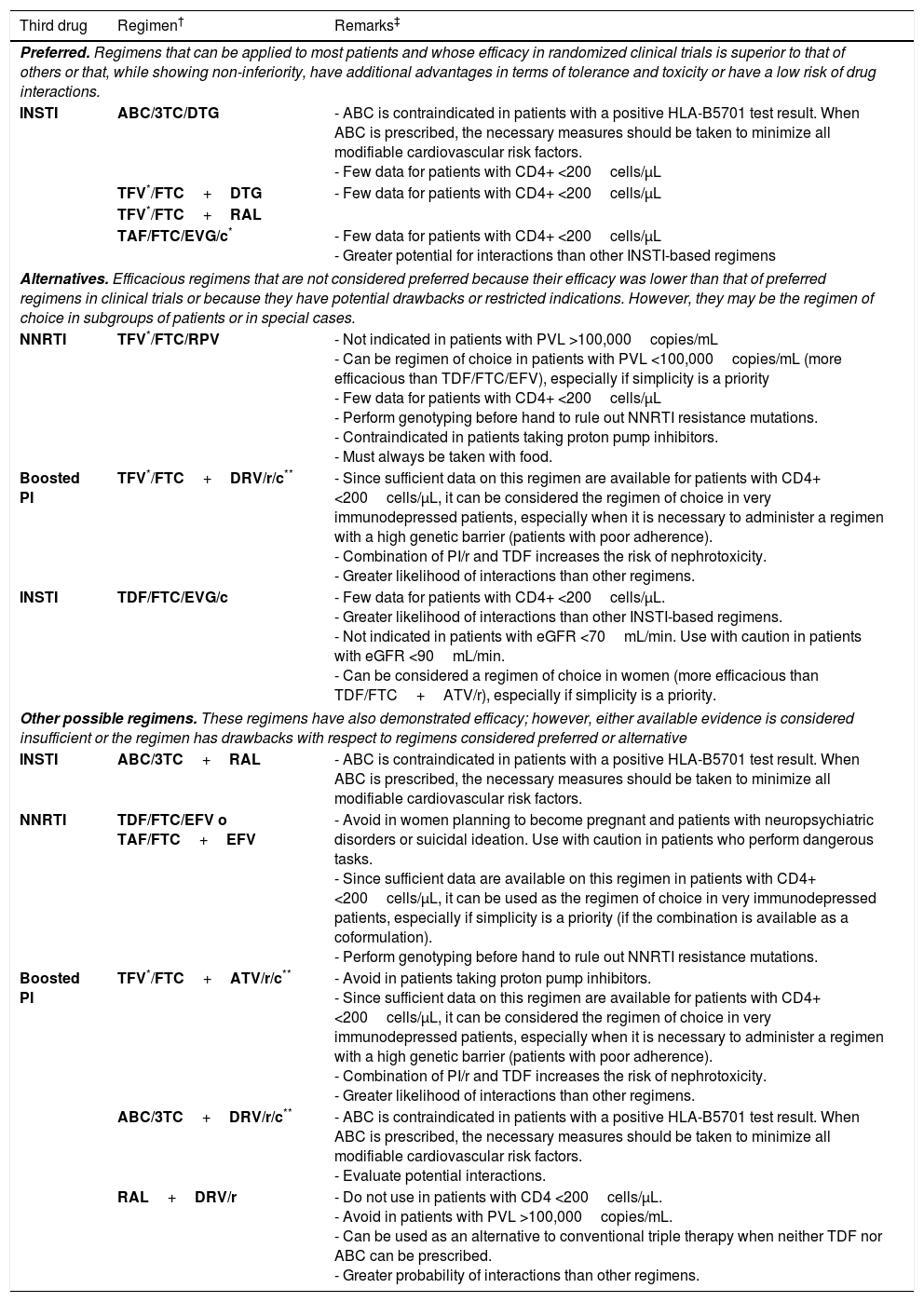
Initial ART can be a combination of 2 nucleoside reverse transcriptase inhibitors (NRTI) and 1 INSTI, 2 NRTI and 1 NNRTI, or 2 NRTI and 1 boosted protease inhibitor (PI) (A-I). Preferred antiretroviral drugs are set out in Table 1.
Recommended combinations of initial ART†
| Third drug | Regimen† | Remarks‡ |
|---|---|---|
| Preferred. Regimens that can be applied to most patients and whose efficacy in randomized clinical trials is superior to that of others or that, while showing non-inferiority, have additional advantages in terms of tolerance and toxicity or have a low risk of drug interactions. | ||
| INSTI | ABC/3TC/DTG | - ABC is contraindicated in patients with a positive HLA-B5701 test result. When ABC is prescribed, the necessary measures should be taken to minimize all modifiable cardiovascular risk factors. - Few data for patients with CD4+ <200cells/μL |
| TFV*/FTC+DTG | - Few data for patients with CD4+ <200cells/μL | |
| TFV*/FTC+RAL | ||
| TAF/FTC/EVG/c* | - Few data for patients with CD4+ <200cells/μL - Greater potential for interactions than other INSTI-based regimens | |
| Alternatives. Efficacious regimens that are not considered preferred because their efficacy was lower than that of preferred regimens in clinical trials or because they have potential drawbacks or restricted indications. However, they may be the regimen of choice in subgroups of patients or in special cases. | ||
| NNRTI | TFV*/FTC/RPV | - Not indicated in patients with PVL >100,000copies/mL - Can be regimen of choice in patients with PVL <100,000copies/mL (more efficacious than TDF/FTC/EFV), especially if simplicity is a priority - Few data for patients with CD4+ <200cells/μL - Perform genotyping before hand to rule out NNRTI resistance mutations. - Contraindicated in patients taking proton pump inhibitors. - Must always be taken with food. |
| Boosted PI | TFV*/FTC+DRV/r/c** | - Since sufficient data on this regimen are available for patients with CD4+ <200cells/μL, it can be considered the regimen of choice in very immunodepressed patients, especially when it is necessary to administer a regimen with a high genetic barrier (patients with poor adherence). - Combination of PI/r and TDF increases the risk of nephrotoxicity. - Greater likelihood of interactions than other regimens. |
| INSTI | TDF/FTC/EVG/c | - Few data for patients with CD4+ <200cells/μL. - Greater likelihood of interactions than other INSTI-based regimens. - Not indicated in patients with eGFR <70mL/min. Use with caution in patients with eGFR <90mL/min. - Can be considered a regimen of choice in women (more efficacious than TDF/FTC+ATV/r), especially if simplicity is a priority. |
| Other possible regimens. These regimens have also demonstrated efficacy; however, either available evidence is considered insufficient or the regimen has drawbacks with respect to regimens considered preferred or alternative | ||
| INSTI | ABC/3TC+RAL | - ABC is contraindicated in patients with a positive HLA-B5701 test result. When ABC is prescribed, the necessary measures should be taken to minimize all modifiable cardiovascular risk factors. |
| NNRTI | TDF/FTC/EFV o TAF/FTC+EFV | - Avoid in women planning to become pregnant and patients with neuropsychiatric disorders or suicidal ideation. Use with caution in patients who perform dangerous tasks. - Since sufficient data are available on this regimen in patients with CD4+ <200cells/μL, it can be used as the regimen of choice in very immunodepressed patients, especially if simplicity is a priority (if the combination is available as a coformulation). - Perform genotyping before hand to rule out NNRTI resistance mutations. |
| Boosted PI | TFV*/FTC+ATV/r/c** | - Avoid in patients taking proton pump inhibitors. - Since sufficient data on this regimen are available for patients with CD4+ <200cells/μL, it can be considered the regimen of choice in very immunodepressed patients, especially when it is necessary to administer a regimen with a high genetic barrier (patients with poor adherence). - Combination of PI/r and TDF increases the risk of nephrotoxicity. - Greater likelihood of interactions than other regimens. |
| ABC/3TC+DRV/r/c** | - ABC is contraindicated in patients with a positive HLA-B5701 test result. When ABC is prescribed, the necessary measures should be taken to minimize all modifiable cardiovascular risk factors. - Evaluate potential interactions. | |
| RAL+DRV/r | - Do not use in patients with CD4 <200cells/μL. - Avoid in patients with PVL >100,000copies/mL. - Can be used as an alternative to conventional triple therapy when neither TDF nor ABC can be prescribed. - Greater probability of interactions than other regimens. | |
ABC, abacavir; ATV/r/c, atazanavir boosted with ritonavir or cobicistat; c, cobicistat; DTG, dolutegravir; DRV/r/c, darunavir boosted with ritonavir or cobicistat; DRV/r, darunavir boosted with ritonavir; eGFR, estimated glomerular filtration rate; EVG/c, elvitegravir boosted with cobicistat; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitors; RAL, raltegravir; RPV, rilpivirine; TFV, tenofovir in either of its 2 formulations; TDF, tenofovir-disoproxil fumarate; TAF, tenofovir-alafenamide; 3TC, lamivudine.
When available, fixed-dose combinations should be used. There are no data showing that FTC and 3TC can be considered therapeutically equivalent; therefore, use of one or other drug in the regimens selected essentially depends on experience of use in combination with other drugs in the regimen.
The clinical trials on which the evidence for each regimen is based are referenced in the text.
In drugs from the same family and with the same level of recommendation, the order reflects the preference of the expert panel.
The remarks reflect aspects that should be taken into consideration when choosing the regimen; they do not aim to be an exhaustive guide to the precautions to be taken when receiving these drugs. Please see the main text and the appropriate Summary of Product Characteristics for more information.
Cost and pricing of the therapeutic regimens are addressed elsewhere in these guidelines. The cost-effectiveness of the regimens is analyzed formally in an article published simultaneously with the guidelines.
TFV can be used as TDF or as TAF. Both formulations have shown equivalent efficacy. TDF should not be used if the eGFR is <50ml/min. TAF is preferred in patients with altered renal function or osteoporosis or who are at risk of these conditions. The coformulations TAF/FTC and TAF/FTC/RPV have been approved by the EMA, although at the time of writing, they are not sold in Spain.
Recommendations
- •
The NRTI combinations of choice for initial regimens are TAF/FTC or TDF/FTC and ABC/3TC (AI).
- •
Co-formulated preparations are recommended (A-II).
- •
The combination TDF/FTC should be avoided in patients with renal insufficiency (A-II).
- •
The combination ABC/3TC should be avoided in patients with a high PVL (>100,000copies/mL) when combined with an NNRTI or a boosted PI (A-II).
Recommendations
- •
The combinations rilpivirine (RPV)/TAF/FTC and RPV/TDF/FTC are considered preferential in patients with a PVL <100,000copies/mL, (A-I).
- •
RPV should not be administered to patients with a PVL >100,000copies/mL (A-II).
- •
Efavirenz (EFV) is contraindicated during the first trimester of pregnancy. Other options are recommended in women who do not use effective contraception. Similarly, EFV should be avoided in patients with neuropsychiatric disorders or a history of suicidal ideation and in patients who perform dangerous tasks if they present symptoms of somnolence, dizziness, and/or difficulty concentrating (A-III).
Recommendations
- •
When it is deemed appropriate to initiate a PI-based regimen, the recommendation is for DRV/r or DRV/c+TDF/FTC or TAF/FTC (QD) (A-I). Alternatively, ATV/r or ATV/c+TDF/FTC or TAF/FTC (QD) could be prescribed (A-I).
- •
ATV and DRV can be boosted interchangeably with ritonavir 100mg or cobicistat 150mg (B-II).
- •
The combination DRV/r or DRV/c+ABC/3TC can also be used, although it has not been formally assessed in a clinical trial (B-III).
- •
DRV/r+RAL can be used as an alternative to conventional triple therapy when it is not possible to use TAF, TDF or ABC (B-I). This regimen should not be used as initial treatment in patients with advanced disease (CD4+ T-lymphocyte counts <200cells/μL and/or a PVL >100,000copies/mL) (A-I).
Recommendations
- •
Dolutegravir (DTG) combined with TDF/FTC or TAF/FTC or coformulated with ABC/3TC, elvitegravir (EVG) coformulated with cobicistat/TAF/FTC (EVG/c/TAF/FTC), and raltegravir (RAL) combined with TDF/FTC or TAF/FTC are considered preferred regimens for initial treatment (A-I).
- •
The combination EVG/c/TAF/FTC is preferred over EVG/c/TDF/FTC owing to its better tolerability profile and the possibility of administering it with an estimated glomerular filtration rate (eGFR) >30mL/min (A-I).
- •
The combination EVG/c/TDF/FTC can be prescribed as an alternative, although not in patients with an estimated glomerular filtration rate <70mL/min) (A-I).
There are several reasons for changing an efficacious ART regimen (e.g., tolerance, toxicity, comorbid conditions, drug interactions, and reducing the pill burden or number of daily doses).
After switching ART in this context, maintenance of virological suppression and performance of relevant laboratory tests should be evaluated within 3–6 weeks.
Remarks on the new regimenRecommendation
- •
In the case of patients with an undetectable PVL, the new regimen should give priority to the drugs recommended as preferential for naïve patients (A-III). In specific cases, alternative regimens, or regimens classed as “other antiretroviral regimens” (Table 1) may be appropriate (A-II).
Recommendation
- •
Switching from a regimen containing 2 NRTI+PI/r to one containing 2 NRTI+1 NNRTI, 1 INSTI or unboosted ATV is only possible if the antiviral activity of the 2 NRTI and third drug can be guaranteed (A-I).
(a) NRTI
Switching from ABC/3TC to TDF/FTCRecommendation
- •
The association between ABC and increased incidence of cardiovascular events is open to debate. This committee cannot make a recommendation on the strength of evidence for switching from ABC/3TC to TDF/FTC (C-I).
Recommendation
- •
The switch from TDF to ABC is a valid option in patients with osteopenia or osteoporosis associated with TDF (A-II).
Recommendation
- •
Switching from TDF/FTC to TAF/FTC is virologically safe. This switch is associated with improved bone mineral density and kidney function (A-I).
(b) NNRTI
Switching from EFV/TDF/FTC to RPV/TDF/FTCRecommendation
- •
In patients with adverse central nervous system (CNS) effects caused by EFV/TDF/FTC, the switch to RPV/TDF/FTC is one of the options that can improve the symptoms associated with EFV (A-II). There are no data in favor of recommending a proactive switch in patients who do not have CNS symptoms or data comparing this switch with a switch to other antiretroviral drugs that do not cause CNS effects.
Recommendation
- •
Switching from RPV/TDF/FTC or EFV/TDF/FTC to RPV/TAF/FTC is virologically safe. This switch is associated with improved bone mineral density and kidney function (A-I).
Recommendation
- •
Switching to EFV/TDF/FTC is an option in patients taking ART with EFV and NVP who wish to reduce their pill burden (A-II).
(c) Protease inhibitors
Switching from ATV/r+ABC/3TC to unboosted ATV+ABC/3TCRecommendation
- •
In patients taking ATV/r+ABC/3TC, switching to ATV+ABC/3TC is a simplification option when attempting to avoid RTV, owing to hyperbilirubinemia, dyslipidemia, diarrhea, or the risk of interactions with RTV (A-I).
Recommendation
- •
In patients receiving treatment with ATV/r or DRV/r, switching to ATV/c (A-I) or DRV/c (A-II) is a simplification option that reduces the pill burden. The results of bioequivalence studies lead this Committee to recommend ATV/c or DRV/c interchangeably in contexts that affect ATV/r or DRV/r as a component of triple regimens (see elsewhere in this chapter). Data on dual regimens or monotherapy are not sufficient to recommend using the drugs interchangeably. Potential interactions with other drugs should always be taken into account, since these are not identical with ritonavir and with cobicistat.
Recommendation
- •
In patients taking ATV/r+TDF/FTC, switching to ATV+ABC/3TC is an option in those cases where both TDF and RTV have to be avoided (B-I).
(a) Switching from NRTI to INSTI
Switching from TDF to RALRecommendation
- •
Switching from TDF to RAL in patients who are also taking a PI/r is also an option in patients with reduced bone mineral density (B-II).
(b) Switching from NRTI to MVC
Recommendation
- •
Switching to a boosted PI and MVC from regimens that contain 1 boosted PI and 2 NRTI is not virologically safe, although genotyping of proviral DNA shows that the virus is R5-tropic. This switch cannot be recommended (A-I).
(c) Switching from NNRTI to INSTI
Switching from EFV to RALRecommendations
- •
Switching from EFV to RAL is an option in patients with CNS adverse events caused by EFV (A-II). There are no data to recommend a proactive change in patients with no CNS symptoms or data or data comparing this switch with a switch to other antiretroviral drugs that do not cause CNS effects.
- •
Switching from EFV to RAL is a valid option in patients with dyslipidemia caused by EFV (A-I).
Recommendation
- •
Switching from TDF/FTC+EFV or NVP to coformulated EVG/c/FTC/TDF is virologically safe. This change is an option for patients who wish to simplify their current regimen and can improve CNS symptoms caused by EFV (A-I). There are no data to recommend a proactive change in patients who do not have CNS symptoms. Similarly, there are no data comparing this switch with switches to other drugs that do not cause CNS symptoms.
(d) Switching from boosted PI to an NNRTI
Switching from a boosted PI to EFV/FTC/TDFRecommendation
- •
Switching to EFV/FTC/TDF is an option in patients who are taking ART with boosted PI. This approach makes it possible to reduce the daily pill burden, although patients may experience EFV-induced CNS adverse effects (B-I).
Recommendation
- •
Switching to an ART regimen comprising 2 NRTI and 1 boosted PI to the co-formulation RPV/FTC/TDF is a valid option in patients with gastrointestinal disorders or dyslipidemia. It also enables the daily pill burden to be reduced (A-I).
(e) Switching from boosted PI to INSTI
Switching from boosted PI to RALRecommendation
- •
Switching to RAL+2 active NRTI is a valid option for patients with dyslipidemia taking ART with NRTI+1 boosted PI (B-I).
Recommendation
- •
Switching from TDF/FTC+ATV/r or DRV/r or LPV/r to EVG/c/TDF/FTC is virologically better than the previous options. This switch is an option for patients who wish to simplify their current regimen and can improve RTV-associated digestive symptoms in some patients (A-I).
(f) Switching to EVG/c/FTC/TAF from TDF-containing regimens
Recommendation
- •
Switching from EVG/c/FTC/TDF, EFV/FTC/TDF, or ATV/r+FTC/TDF to EVG/c/FTC/TAF is virologically safe in patients whose virus remains sensitive to all the components in the regimen. This change is also associated with improved bone mineral density and kidney function. The switch is even feasible in patients with mild or moderate kidney failure (A-I).
(g) Switching to DTG/ABC/3TC from regimens containing 2 NRTI and PI, NNRTI, or INSTI
Recommendation
- •
Switching to DTG/ABC/3TC, from regimens containing 2 NRTI and PI, NNRTI, or INSTI is virologically safe. This switch is an option in patients who wish to simplify their current regimen.
(h) Switch from a boosted PI to MVC
Recommendation
- •
Switching to 2 NRTI and MVC from regimens that contain 2 NRTI and PI is virologically safe if genotyping of proviral DNA shows that the virus is R5-tropic (A-I)
Recommendation
- •
Switching from 2 NRTI+ATV/r or DRV/r or LPV/r to dual therapy with 3TC+ATV/r or 3TC+DRV/r or 3TC+LPV/r is an option if the clinician wishes to avoid or prevent the adverse effects caused by NRTI. This option requires the patient to fulfill the following criteria: (1) No chronic hepatitis B; (2) PVL <50copies/mL for at least 6 months; and (3) No mutations in the protease gene or previous virological failure to PI/r or 3TC (A-I).
Recommendation
- •
Monotherapy with DRV/r once daily or LPV/r twice daily is a valid option for treating or preventing adverse effects caused by NRTI if the patient fulfills the following criteria: (1) No chronic hepatitis B; (2) PVL <50copies/mL for at least 6 months; (3) No mutations in the protease gene and no previous virological failure with PI (B-I). Since there are no data on the efficacy of monotherapy with DRV/c, this regimen cannot be recommended at present. Given that monotherapy with DRV/r or LPV/r carries a greater risk of viral rebound than dual therapy with DRV/r or LPV/r with 3TC, this Committee recommends the use of monotherapy only in unusual cases where dual therapy cannot be used.
Definition of virological failure (VF). Two confirmed determinations of PVL >50copies/mL 24 weeks after initiating ART.
Recommendations
- •
The objective of rescue ART is to achieve a PVL <50copies/mL (A-II).
- •
Switching ART because of VF should be performed early to avoid accumulation of mutations and to facilitate the response to the new treatment (A-III).
- •
The new ART regimen should contain 3 totally active antiretroviral drugs. If this is not possible, 2 fully active drugs should be combined with other drugs that maintain partial virological activity, especially in the case of advanced rescue in patients with limited therapeutic options (A-I). Regimens with only 2 active antiretroviral drugs based on a boosted PI may be a reasonable option in patients who have experienced a non-advanced failure when it is not possible to use NRTI or construct a simple regimen with 3 active drugs (A-I).
- •
Resistance and viral tropisms should be assessed in order to design the best alternative regimen. The test should be performed while the patient is receiving the failed treatment or as soon as possible after suspension of the failed treatment. If the results of previous genotyping tests are available, all the resistance mutations detected should be evaluated (A-I).
- •
The causes of VF—poor adherence, drug or food interactions, previous intolerance and previous toxicity—should be analyzed. The new regimen should be comfortable and well tolerated (A-III).
- •
In patients who have experienced VF, DRV/r is the PI/r that has proven most efficacious in all the rescue lines. When major resistance mutations are present, the recommended dose is 600/100mg BID (A-I).
- •
DTG is the INSTI of choice in patients who experience VF who are INSTI-naïve (A-I). In the case of previous failure to RAL or EVG, the recommended dose of DTG is 50mg BID, accompanied by optimized background therapy (A-II).
- •
The use of tipranavir/ritonavir (TPV/r), enfuvirtide, or thymidine analogs is restricted to patients with no other therapeutic options (A-III).
- •
In patients with low-grade VF (PVL detectable but ≤200copies/mL), genotyping can be performed with a 2–3–mL plasma sample (A-II). If genotyping does not reveal resistance mutations, an ART regimen with a high barrier to resistance should be maintained. In patients with a PVL >200copies/mL, genotyping should be performed. The choice of the new ART regimen should be based on both resistance mutations and previous ART. ART should not be intensified with a single drug (A-III).
- •
ART should not be suspended in patients with advanced VF and no therapeutic options (A-II). In this situation, the approach should involve antiretroviral drugs that reduce viral replicative capacity and do not lead to resistance mutations that might compromise future treatments (A-III).
- •
In patients with no therapeutic options, it is important to monitor the CD4+ count and PVL and to consult with clinicians and virologists specialized in resistance and rescue therapy who are involved in restricted access programs (B-III).
Recommendations
- •
Before initiating ART, the patient should be prepared and factors likely to limit adherence should be identified and corrected (A-III).
- •
Once ART has been initiated, a first check-up should be made after 2–4 weeks to verify adherence and correct adherence problems if necessary (A-III).
- •
Adherence should be monitored and reinforced at visits to the doctor (A-III).
- •
Adherence should be monitored by a multidisciplinary team including a doctor, nursing staff, specialists in psychological support, and a hospital pharmacist (A-III).
- •
In the case of patients whose adherence is irregular, it is recommended to use regimens based on boosted PI, preferably DRV because of its high genetic barrier to resistance, in order to prevent the development of resistance (A-II).
- •
Using fixed dose combinations of antiretroviral drugs simplifies ART and thus facilitates continued adherence. The use of whole regimens in a single tablet is the most efficient strategy for preventing selective poor adherence (A-II).
Recommendations
- •
Avoid the use of antiretroviral drugs whose immediate adverse effects are similar to clinical manifestations or laboratory abnormalities that are already present in a specific patient (A-II).
- •
HLA-B*5701 testing is mandatory before prescribing ABC, since it has a negative predictive value of almost 100% for the risk of hypersensitivity reaction to this drug (A-I).
- •
If the adverse effect is very intense or long-lasting or cannot be tolerated by the patient, the potential culprit antiretroviral drug(s) should be switched (A-I).
Recommendations
- •
ART should be tailored by evaluating the risk or presence of chronic diseases in such a way that the regimen selected does not contain antiretroviral drugs that can favor the onset or progression of these diseases (A-II).
- •
Withdrawal of some of the antiretroviral drugs involved in late adverse effects can improve—albeit partially—the underlying clinical abnormality, although it is not known whether such a modification can alter the natural history of the specific chronic disease or survival. Antiretroviral drugs contribute collaterally to the risk or progression of specific chronic diseases, although other factors are generally considered to be more important. Priority should be given to interventions to address these factors (A-II).
Recommendations
- •
All medications, natural products, alternative medicines and recreational drugs taken by the patient should be recorded in the clinical history in order to evaluate potential interactions (A-III).
- •
Contraindications should be taken into account and the corresponding dose adjustments made where necessary (A-I).
- •
Plasma levels should be monitored when prescribing two or more drugs with potential pharmacokinetic interactions in order to avoid toxicity or lack of efficacy (A-II).
Recommendations
- •
ART should be recommended in all patients with acute HIV infection, regardless of the symptoms, their severity, or their duration (A-II) and should be started as soon as possible to obtain the maximum benefit.
- •
If ART is to be initiated, it should be done so with the same preferential regimens used to treat chronic HIV infection (A-I) (Table 1). A regimen comprising 2 NRTI (preferably TAF-TDF/FTC) and an INSTI could reduce PVL more rapidly during the first 4–8 weeks than PI or NNRTI and, thus, make it easier to reduce transmission of HIV (A-I) and reach higher concentrations in genital tract secretions (B-III).
- •
If the results of resistance testing are not available, it is preferable to begin with a regimen based on DTG or boosted DRV until the results become available (A-II).
- •
If is ART is initiated, it should be administered indefinitely (A-I).
Recommendations
- •
The general principles of ART in patients infected by HIV-2 should be the same as those of HIV-1 infection (A-III). Clinical monitoring is recommended. The CD4+ lymphocyte count should be determined every 3–6 month, as should HIV-2 PVL, if available.
- •
The preferred regimen for initial ART in these patients is the combination of 2 NRTI and 1 INSTI or a boosted PI (A-III).
- •
The use of NNRTI, MVC, or ENF is not indicated for the treatment of HIV-2 infection (A-I).
A specific GESIDA document is available. The most important recommendations are summarized below.
Recommendations
- •
All pregnant women must undergo HIV serology testing (A-I). If the result is negative, testing must be repeated during the third trimester (A-II).
- •
Pre-pregnancy counseling must form part of health care for HIV-infected women of childbearing age and should include a recommendation for ART so that the woman can become pregnant with an undetectable PVL (A-II).
- •
ART is indicated in all pregnant women, irrespective of CD4+ T-lymphocyte count and PVL, in order to ensure that PVL remains undetectable for as long as possible during pregnancy, especially during the third trimester and at delivery (A-I).
- •
The choice of specific antiretroviral drugs should be based on resistance studies, drug safety, and ease of adherence. If there are no resistance mutations, the regimen of choice is TDF or ABC+3TC or FTC+LPV/r or ATV/r (A-I) or DRV/r (A-II) or RAL (A-II); if resistance mutations are detected, patients can receive any of the “preferential” and “alternative” antiretroviral drugs after a personalized evaluation (A-III).
- •
Intrapartum intravenous administration of ZDV is only indicated in women whose PVL is >1000copies/mL or unknown at the time of delivery, irrespective of any previous ART received (A-I).
- •
Elective cesarean delivery is indicated at week 38 in women with a pre-labor PVL of >1000copies/mL (A-II).
- •
Mothers cannot breastfeed. Adapted formula food must be used (A-I).
Recommendations
- •
In most opportunistic infections (except tuberculosis and cryptococcal meningitis), ART should be started as soon as possible (preferably within the first 15 days after starting treatment for the infection) (A-II).
- •
Patients with Pneumocystis jiroveci pneumonia who are not receiving ART, should start ART during the 2 weeks following the diagnosis of Pneumocystis jiroveci pneumonia (A-I).
- •
In patients with cryptococcal meningitis, initiation of ART should be deferred for 5 weeks because of the greater risk of death associated with early initiation (especially in patients with <5cells/μL in CSF or increased intracranial pressure) (A-I).
Treatment of tuberculosis in HIV-infected adults was the subject of a consensus document from GESIDA/National AIDS Plan, which is available for consultation.
Optimal timing of ARTRecommendations
- •
ART should always be started during treatment of tuberculosis, irrespective of the CD4+ T-lymphocyte count, since it reduces the risk of death (A-I).
- •
The optimal time for initiating ART depends on the CD4+ T-lymphocyte count. If the CD4+ T-lymphocyte count is <50cells/μL, ART should be started as soon as possible, after verifying tolerance to anti-tuberculosis treatment, but not later than the first 2 weeks (A-I). If the CD4+ T-lymphocyte count is >50cells/μL, initiation of ART can be delayed until the intense phase of anti-tuberculosis treatment has been completed (8 weeks). This approach reduces the risk of adverse effects and the development of immune reconstitution inflammatory syndrome (IRIS) without compromising survival (A-I).
Recommendations
- •
Choice of NRTI: No significant interactions or evidence of toxicity have been found between antituberculosis drugs and NRTI. Therefore, ABC, TDF, 3TC, and FTC can be used in these patients with no added risks (A-I). However, a relevant interaction could occur between TAF and the rifamycins, with a decrease in absorption and in the plasma concentration of TAF, since TAF is transported by glycoprotein P (P-gp) and the rifamycins induce the activity of this protein.
- •
Choice of the third drug. Since most experience and the best results have been obtained with EFV, this is the antiretroviral drug of choice (A-I). The dose of EFV is standard for all patients (600mg/d), irrespective of body weight and with no need to increase to 800mg/d (A-I).
- •
Alternative third drugs. Based on experience or sufficient evidence, the alternative regimens that can be recommended include NVP at habitual doses (A-II) and RAL at 800mg/12h (A-II), although 400mg/12h has proven to be efficacious, as has MVC at 600mg/12h (A-III). Despite the absence of clinical data, the results of pharmacokinetic studies show that DTG can be administered at 50mg/12hours (A-III).
- •
Drugs that cannot be used. The other NNRTI (RPV and ETV), PI (whether boosted or not with ritonavir or cobicistat), and EVG should not be co-administered with rifampicin. In the exceptional case of a PI being the only option for ART, rifampicin should be replaced by rifabutin and the corresponding adjustment in drug doses should be made (A-II).
Recommendations
- •
If the patient develops IRIS, neither ART nor anti-tuberculosis medication should be interrupted (A-III).
- •
The symptoms of IRIS can by managed by adding non-steroidal anti-inflammatory drugs in mild to moderate cases (A-III) or corticosteroids in moderate to severe forms (A-II).
For a complete overview of renal disorders in HIV-infected patients, please consult the consensus document drafted by GESIDA, the SEN, and the SEQC.
Recommendations
- •
It is necessary to adjust the dose of NRTI, except for ABC (A-II).
- •
No dose adjustment is required for NNRTI, PI, ENF, RAL, or DTG (A-II).
- •
The dose of MVC should be adjusted if it is used in combination with potent CYP3A4 inhibitors such as PI (except TPV/r), ketoconazole, itraconazole, clarithromycin, and telithromycin (A-II).
- •
Co-formulations of antiretroviral drugs are not advised in patients with significant renal insufficiency. The co-formulation EVG/c/FTC/TDF should not be used in patients with an eGFR <70mL/min. The co-formulations EFV/FTC/TDF, RPV/EFV/TDF, and DTG/ABC/3TC should not be used in patients with eGFR <50mL/min. The co-formulation EVG/c/FTC/TAF should not be used in patients with eGFR <30mL/min. In these cases, antiretroviral drugs should be administered separately and the appropriate adjustments made (B-III).
- •
In patients with renal insufficiency (any stage), kidney function should be closely monitored and nephrotoxic drugs avoided (A-III).
- •
In patients with advanced chronic renal insufficiency, the dose should be adjusted according to the recommendations of the summary of product characteristics, taking into account possible drug interactions, which are more common and more dangerous in this situation (A-II). In the absence of contraindications, the combination of ABC+3TC (adjusted for eGFR) with an NNRTI or a non-boosted INSTI (DTG or RAL) or DRV/r can be used (A-III).
Both SEIMC and AEEH recently drafted guidelines for the management of hepatitis C. Please consult the guidelines for more detailed information.
Initiation of ARTRecommendations
- •
Patients co-infected with HCV should initiate ART irrespective of their CD4+ T lymphocyte count (A-I).
- •
In patients who require treatment for hepatitis C, it is generally preferable to initiate ART before starting treatment for HCV infection A-III).
- •
Patients co-infected with HBV for whom treatment of HBV infection is indicated should initiate ART containing TDF or TAF and FTC or 3TC (A-I).
Recommendations
- •
Any antiretroviral drug can be used in patients with chronic liver disease and normal liver function, including patients with cirrhosis (Child-Pugh, class A) (A-I), although it seems reasonable to avoid dideoxynucleoside drugs and nevirapine (A-III).
- •
In patients with mild or moderate hepatocellular insufficiency (Child–Pugh stage A or B), INSTI do not require dose adjustments and are the drugs of choice (A-I). Boosted PI have a greater therapeutic margin than NNRTI (A-II). In patients with Child–Pugh stage C disease, RAL does not require dose adjustment and ATV/r and FPV/r (with the dose of FPV/r adjusted) are safe (A-II).
- •
With the exception of sofosbuvir, currently used DAA (simeprevir, daclatasvir, ledipasvir, paritaprevir, ombitasvir, dasabuvir, elbasvir and grazoprevir) present significant pharmacokinetic interactions with antiretroviral drugs that may require doses to be adjusted or coadministration to be contraindicated (A-I).
- •
An updated pharmacologic interaction software package should be used before prescribing a DAA-containing regimen in a patient on ART (A-III).
Please refer to the relevant GESIDA documents for complete information on cancer in HIV-infected patients.
Recommendations
- •
ART is an essential component of the treatment of HIV-infected patients with Kaposi sarcoma or non-Hodgkin lymphoma (A-II).
- •
Patients with other types of cancer who are not receiving ART should initiate therapy as soon as possible (A-II).
- •
Given its pharmacological characteristics, excellent tolerance, and minimal interactions, RAL should be the antiretroviral drug of choice, where possible, in patients receiving chemotherapy (A-III). DTG can be considered an alternative in cases of resistance to RAL (C-III).
Together with the present consensus document, GESIDA has published a pharmaco-economic study in which the cost-effectiveness of the recommended preferred and alternative regimens is evaluated. Please consult the relevant document.
Recommendation
- •
Cost-effectiveness criteria should be taken into account when deciding on initial ART (A-III).
This consensus document was drafted without grant aid or other funding, whether collective or individual, from any private institutions. The conflicts of interest not associated with this document declared by the Members of the Editorial Board are set out below.
Antonio Antela has received payment for participating in consultancy meetings, acting as a researcher in clinical trials, and speaking at meetings or symposia from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare.
José R. Arribas has acted as a consultant for AbbVie Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Tobira, and ViiV Healthcare. He has received grant support for clinical research from Janssen, Merck Sharp & Dohme, and Gilead Sciences, and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Victor Asensi has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has also received research grants from Janssen.
Juan Berenguer has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Janssen Therapeutics, Merck Sharp & Dohme, and ViiV Health care. He has received clinical research grant support from Bristol-Myers Squibb, Merck Sharp & Dohme, and ViiV Health care and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Health care.
José R. Blanco has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from Bristol-Myers Squibb y Gilead Sciences, and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
José Luis Casado has acted as a consultant for AbbVie and ViiV Healthcare. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received clinical research grants from Janssen, Gilead Sciences, and ViiV Healthcare.
Bonaventura Clotet has acted as a consultant, participated in clinical trials, and given paid talks for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Manuel Crespo has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received clinical research grants from Gilead Sciences and ViiV Healthcare. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and for developing training presentations from Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare.
Pere Domingo has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare and payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare.
Carlos Dueñas has acted as a consultant for Gilead Sciences and Janssen. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
José M. Gatell has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare.
Juan Luis Gómez Sirvent has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb and Merck Sharp & Dohme. He has also participated in clinical trials and studies sponsored by AbbVie, Boehringer Ingelheim, Gilead Sciences, Janssen, and ViiV Healthcare and has received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Juan González García has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. Has received clinical research grant support and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has also received financial support to attend scientific meetings from Bristol-Myers Squibb, Gilead Sciences and Janssen.
José Antonio Iribarren has acted as a consultant for AbbVie and Janssen, and has received clinical research grant support from AbbVie, Bristol-Myers Squibb, Gobierno Vasco, FIPSE, and FISS. He has also received financial support to attend scientific meetings from AbbVie, Gilead Sciences, Janssen, and ViiV, and has participated in training activities, talks, and symposia sponsored by AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Novartis, Janssen, Pfizer, and ViiV.
José López Aldeguer has acted as a consultant for Gilead Sciences, Janssen, and ViiV Healthcare. He has received clinical research grant support from ViiV Healthcare and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Juan C. López Bernaldo de Quirós has acted as a consultant for Janssen and ViiV Healthcare and has received lecture fees from AbbVie, Bristol-Myers Squibb, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Luis F. López Cortés has received unrestricted research funding, consultancy fees, and lecture fees from and have served on the advisory boards of Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Juan E. Losa has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Fernando Lozano has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dohme, and ViiV Healthcare and has received payment for training sessions from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dohme, and ViiV Healthcare.
Josep Mallolas has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare and has received research grants from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare. He has also received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare.
Ana Mariño has received grant aid for attendance at conferences and meetings from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare.
Esteban Martínez has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, and ViiV Healthcare. He has also received research grants from Merck Sharp & Dohme and payment for training sessions from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, MerckSharp & Dohme, and ViiV Healthcare.
José M. Miró has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Novartis, and Sanofi and has received clinical research grant support from Cubist, Gilead Sciences, Merck Sharp & Dohme, Novartis, Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (Madrid), Fundación para la Investigación y Prevención del Sida en España (FIPSE, Madrid), Ministerio de Sanidad, Servicios Sociales e Igualdad (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA), and NEAT. He has also received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Novartis, and ViiV Healthcare.
Santiago Moreno has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and Roche Pharma, and has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and Roche Pharma. He has also received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and Roche Pharma.
Rosario Palacios has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, and Janssen and has received payment for talks from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen, Gilead Sciences, and ViiV-Healthcare.
Juan Pasquau has received payment during the last 3 years for scientific consultancy services from AbbVie, Bristol-Myers Squibb, Janssen, and ViiV Healthcare and for lectures and other training activities from Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has also received research grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
José A. Pérez Molina has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Bristol-Myers Squibb, Merck Sharp & Dohme, and ViiV Healthcare.
Juan A. Pineda declares that he has received payment for consultancy services from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, and Merck Sharp & Dohme, and research grants from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche Pharma, and ViiV Healthcare. He has also received payment for talks from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche Pharma, and ViiV Healthcare.
Daniel Podzamczer has received research grants and/or consultancy fees and/or lecture fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Rosa Polo declares that she has not received any grant aid or subsidies associated with this document.
Joaquín Portilla has acted as a consultant for AbbVie, Gilead Sciences, and Janssen; he has received payment for talks from AbbVie, Bristol Myers-Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Eva Poveda has received grants for attending conferences and scientific meetings from Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. She has received payment for talks from Janssen Cilag and Merck Sharp & Dohme and grants for developing research projects and biomedical training activities from Gilead Sciences and Janssen.
Federico Pulido has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Antonio Rivero has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare.
Rafael Rubio has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Janssen and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Roche Phasma, and ViiV Healthcare.
Jesús Santos has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV-Healthcare and has received fees for educational talks from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen, Gilead Sciences, and ViiV-Healthcare.
José Sanz Moreno has participated in clinical trials sponsored by Bristol-Myers Squibb, and ViiV Healthcare. He has received payment for consultancy work and preparing training presentations for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Jesús Sanz Sanz has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare, and for preparing training presentations from ViiV Healthcare.
María Jesús Téllez has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Janssen and has received payment for talks from Gilead Sciences and Janssen.
Javier de la Torre has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Montserrat Tuset has received clinical research grant support from Bristol-Myers Squibb, Gilead Sciences, Janssen, and Merck Sharp & Dohme and payment for talks from Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
The Board of GESIDA and the National AIDS Plan acknowledge the contributions and opinions of Marisa Álvarez, Belén Box, Santiago Cenoz, Inmaculada Clotet, Manuel Cotarelo, Adrián Curran, Pedro Ferrer, Beatriz Hernández-Novoa, Marcela González, Cristina González-Conde, Carmen González-Ortega, José Emilio Martín-Herrero, Julián Olalla, Siria Pablos, Óscar Rincón, Felipe Rodríguez-Alcántara, Nuria Sánchez, Ana Serra, Sergio Serrano, José Verdejo, and Eugenia Vispo.
Antonio Rivero1, Rosa Polo2, José López Aldeguer3, Fernando Lozano4, Antonio Antela5, José Ramón Arribas6, Víctor Asensi7, Juan Berenguer8, José Ramón Blanco9, José Luis Casado10, Bonaventura Clotet11, Manuel Crespo12, Pere Domingo13, Carlos Dueñas14, José María Gatell15, Juan Luis Gómez-Sirvent16, Juan González-García17, José Antonio Iribarren18, Juan Carlos López Bernaldo de Quirós19, Luis Fernando López Cortés20, Juan Emilio Losa21, Josep Mallolas22, Ana Mariño23, Esteban Martínez24, José M. Miró25, Santiago Moreno26, Rosario Palacios27, Juan Pasquau28, Juan Antonio Pineda29, Daniel Podzamczer30, Joaquín Portilla31, Eva Poveda32, Federico Pulido33, Rafael Rubio34, Jesús Santos35, José Sanz Moreno36, Jesús Sanz Sanz37, María Jesús Téllez38, Javier de la Torre39, Montserrat Tuset40, José A. Pérez Molina41.
1Hospital Universitario Reina Sofía/IMIBIC, Córdoba; 2Secretaría del Plan Nacional sobre el Sida, Ministerio de Sanidad, Servicios Sociales e Igualdad; 3Hospital Universitario La Fe, IIS La Fe, Valencia; 4Hospital Universitario Virgen de Valme, Sevilla; 5Hospital Clínico Universitario, Santiago de Compostela; 6Hospital Universitario La Paz-IdiPAZ, Madrid; 7Hospital Universitario Central de Asturias, Oviedo; 8Hospital General Universitario Gregorio Marañón, Madrid 9Hospital San Pedro-CIBIR, Logroño; 10Hospital Ramón y Cajal-IRYCIS, Madrid; 11Hospital Germans Trias i Pujol, Badalona; 12Complexo Hospitalario Universitario de Vigo; 13Hospitals Universitaris Arnau de Vilanova i Santa Maria, Universitat de Lleida, IRB-Lleida, Lleida; 14Hospital Universitario de Valladolid (HCUV); 15Hospital Clínic/IDIBAPS, UB, Barcelona; 16Hospital Universitario de Canarias, Santa Cruz de Tenerife; 17Hospital Universitario La Paz/IdiPAZ, Madrid; 18Hospital Universitario Donostia, San Sebastián; 19Hospital General Universitario Gregorio Marañón, Madrid; 20Hospital Universitario Virgen del Rocío, Sevilla; 21Hospital Universitario Fundación Alcorcón, Alcorcón (Madrid); 22Hospital Clínic/IDIBAPS, UB, Barcelona; 23Complejo Hospitalario Universitario de Ferrol; 24Hospital Clínic/IDIBAPS, UB, Barcelona; 25Hospital Clínic/IDIBAPS, UB, Barcelona; 26Hospital Ramón y Cajal/IRYCIS, Madrid; 27Hospital Universitario Virgen de la Victoria, Málaga; 28Hospital Virgen de las Nieves, Granada; 29Hospital Universitario Virgen de Valme, Sevilla; 30IDIBELL-Hospital Universitario de Bellvitge, L’Hospitalet, Barcelona; 31Hospital General Universitario, Alicante; 32Complejo Hospitalario Universitario, A Coruña; 33Hospital Universitario 12 de Octubre, Madrid; 34Hospital Universitario 12 de Octubre, Madrid; 35Hospital Universitario Virgen de la Victoria, Málaga; 36Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid; 37Hospital Universitario de la Princesa, Madrid; 38Hospital Clínico San Carlos, Madrid; 39Hospital Costa del Sol, Marbella; 40Hospital Clínic/IDIBAPS, UB, Barcelona; 41Hospital Ramón y Cajal/IRYCIS, Madrid.