In Venezuela, there have been some reports of carbapenemase KPC-producing Klebsiella pneumoniae. Nevertheless, since the first report in 2008, only a few studies have been done on their molecular epidemiology in this country.
MethodsThe aims of this study were to detect extended-spectrum betalactamase (ESBL)-producing (blaTEM and blaCTM-M-1) and to determine the genetic relationship between 30 isolates of carbapenemase KPC-producing K. pneumoniae taken from patients at eleven health centres in different states of Venezuela from January 2008 to December 2012, using pulsed-field gel electrophoresis (PFGE).
ResultsAll isolates were identified as K. pneumoniae subsp. pneumoniae. Isolates showed the highest resistance to the ertapenem, 97%. The KPC gene was detected in all studied strains. Seventy three percent showed ESBL, having the blaTEM in 68% and blaTEM, CTX-M-1 in 27% of the strains. Eleven groups were found using the field-pulsed gel electrophoresis.
ConclusionHigh genetic diversity was found during 2008–2012 in K. pneumoniae isolated at different states in Venezuela, some of them circulating at eleven health centres. Results showed the importance of performing epidemiologic studies and the need to develop some activities to control this type of microorganisms.
En Venezuela hay reportes de Klebsiella pneumoniae con carbapenemasa tipo KPC. Sin embargo, desde su primer reporte en el 2008, son muy escasos los estudios de epidemiología molecular que se han realizado en estos aislados.
MétodosLos objetivos de esta investigación fueron detectar la producción de betalactamasas de espectro extendido (BLEE) (blaTEM y grupo blaCTX-M-1) y determinar la relación genética de 30 aislados pertenecientes a brotes importantes de K. pneumoniae productores de carbapenemasa tipo KPC derivados por once centros sanitarios de diferentes estados de Venezuela entre enero de 2008 y diciembre de 2012 mediante electroforesis en campo pulsante (ECP).
ResultadosTodos los aislados fueron identificados como K. pneumoniae subsp. pneumoniae. Los aislados mostraron el mayor porcentaje de resistencia al ertapenem, un 97%. En todos los aislados se detectó el gen tipo KPC. El 73% presentó BLEE (en el 68% se detectó blaTEM y en el 27% blaTEM, CTX-M-1). En la ECP se detectaron 11 agrupaciones.
ConclusiónDurante los años 2008-2012 se demostró que existe una gran diversidad genética en los aislados en estudio. Se determinó que algunos aislados circularon en los 11 centros sanitarios. Los resultados de esta investigación plantean la necesidad de fortalecer la vigilancia epidemiológica y el desarrollo de actividades para prevenir y controlar este tipo de microorganismo.
Klebsiella pneumoniae is considered one of the species of the Enterobacteriaceae family that can commonly acquire genes for resistance to carbapenems.1 Between 2001 and 2011, an increase of 1.6–10.4% in the number of K. pneumoniae isolates resistant to carbapenems was reported in the United States, due to the production of KPC-type carbapenemases. It has been reported that patients infected with K. pneumoniae producing KPC-type carbapenemases have a higher mortality than those infected with isolates producing other types of carbapenemases (47.66 and 46.71%, respectively).2 It is considered an unprecedented public health problem.3
The use of molecular techniques such as pulsed-field gel electrophoresis (PFE) has made it possible to evaluate the dissemination of isolates producing KPC-type carbapenemase.4 This technique has a high discriminatory power and good reproducibility, being considered the standard technique of reference for the typification of bacteria.5
Since 2008, the presence of KPC-type carbapenemase-producing K. pneumoniae in Venezuela has been demonstrated, according to the records of the Rafael Rangel National Institute of Hygiene (unpublished data). Subsequently, other reports have been made in the Capital District,6 Carabobo and Zulia7 in coexistence with other determinants of beta-lactam resistance; however, there are no reports of the molecular epidemiology of these early isolates in different states of Venezuela. The objective of this investigation was to detect the production of extended-spectrum beta-lactamases (ESBL) (blaTEM and blaCTX-M-1) and to determine the genetic relationship by PFE in KPC-type carbapenemase K pneumoniae isolates from different health centres in various states of Venezuela from 2008 to 2012.
Materials and methodsIsolates studiedAt the Rafael Rangel National Institute of Hygiene, through the algorithm of the Latin American Surveillance Network of Antimicrobial Resistance,8 the different public and private health centres of the country send strains with unusual resistance phenotypes for confirmation and characterisation. This is how 30 clinical isolates representative of significant hospital outbreaks, not duplicated from different health centres, of KPC-type carbapenemase-producing K pneumoniae stored from 2008 to 2012 were selected in the institution's strain collection. The identification of all the isolates was carried out by means of conventional biochemical tests.
The research complies with the fundamental principles of bioethics and those established in the Declaration of Helsinki of the World Medical Association9 and the international ethical guidelines for biomedical research.10
Study of antibiotic susceptibilityIt was done using the Kirby-Bauer method11 evaluating the discs of imipenem 10μg, meropenem 10μg and ertapenem 10μg (Difco & BBL, USA) according to the cut-off points established in document M100-S25 of the Clinical and Laboratory Standards Institute (CLSI).12 The strains Escherichia coli ATCC® 25922 and Pseudomonas aeruginosa ATCC® 27853 were used as quality controls for antibiotic discs.
Phenotypic characterisation of beta-lactamasesThe detection of carbapenemases was carried out by the use of inhibitors such as 3-aminophenylboronic acid 300μg/ml (Sigma-Aldrich, USA) for the search for serine protease enzymes and ethylenediaminetetraacetic acid/sodium mercaptoacetate 1000mM for the search for metallo-beta-lactamases.6 The detection of ESBL was performed by the double disc confirmatory method.12
To control the activity of the different inhibitors, the following strains were used: K. pneumoniae M13403,13K. pneumoniae M988514 and K. pneumoniae ATCC® 700603.
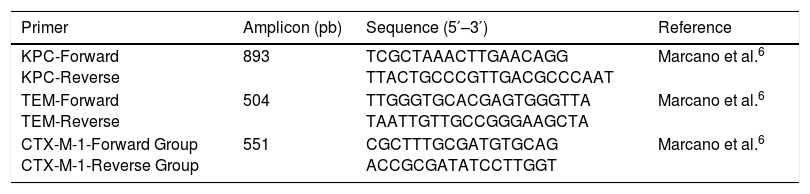
Genotypic characterisation of beta-lactamasesIn all isolates, the gene encoding for KPC-type carbapenemase was amplified. The detection of the genes encoding for the TEM-type beta-lactamase and the CTX-M-1 group was carried out in positive ESBL isolates. The detection was performed using the polymerase chain reaction (PCR) endpoint technique. The initiators used are shown in Table 1.
Primers used for the detection of genes that encode beta-lactamases.
| Primer | Amplicon (pb) | Sequence (5′–3′) | Reference |
|---|---|---|---|
| KPC-Forward KPC-Reverse | 893 | TCGCTAAACTTGAACAGG TTACTGCCCGTTGACGCCCAAT | Marcano et al.6 |
| TEM-Forward TEM-Reverse | 504 | TTGGGTGCACGAGTGGGTTA TAATTGTTGCCGGGAAGCTA | Marcano et al.6 |
| CTX-M-1-Forward Group CTX-M-1-Reverse Group | 551 | CGCTTTGCGATGTGCAG ACCGCGATATCCTTGGT | Marcano et al.6 |
DNA extraction was carried out by the boiling method15 and the amplification and visualisation reactions of the products were carried out according to the protocol used in other studies.6
The positive controls were: K. pneumoniae M1340313 and K. pneumoniae M9885.14
Molecular typificationThe genetic relationship among the 30 isolates studied was determined by PFE. The restriction enzyme XbaI was used as indicated by the standardised protocol of the PulseNet network for E. coli O157, Salmonella spp. and Shigella sonnei.16 The Salmonella serovar Braenderup H9812 (ATCC® BAA-664) was used as the standard.
The results were analysed with the UPGMA (unweighted pair group method with arithmetic mean) method and the Dice coefficient of the Bionumeric program, version 4.0 (Applied Maths, Sint-Martens-Latem, Belgium). The PFE patterns were grouped with a similarity ≥85%.17 In the analysis of the PFE gels, the restriction fragments with sizes between 1135 and 54.7kb were used. The interpretation of the restriction patterns was based on the criteria of Tenover et al.18 When differences greater than or equal to 85% were found in the pulsotype, the isolates were designated as different types and assigned letters (A, B, C, etc.). When one or more fragments were different between the isolates of the same type, they were assigned a subtype, which was designated with Arabic numerals.
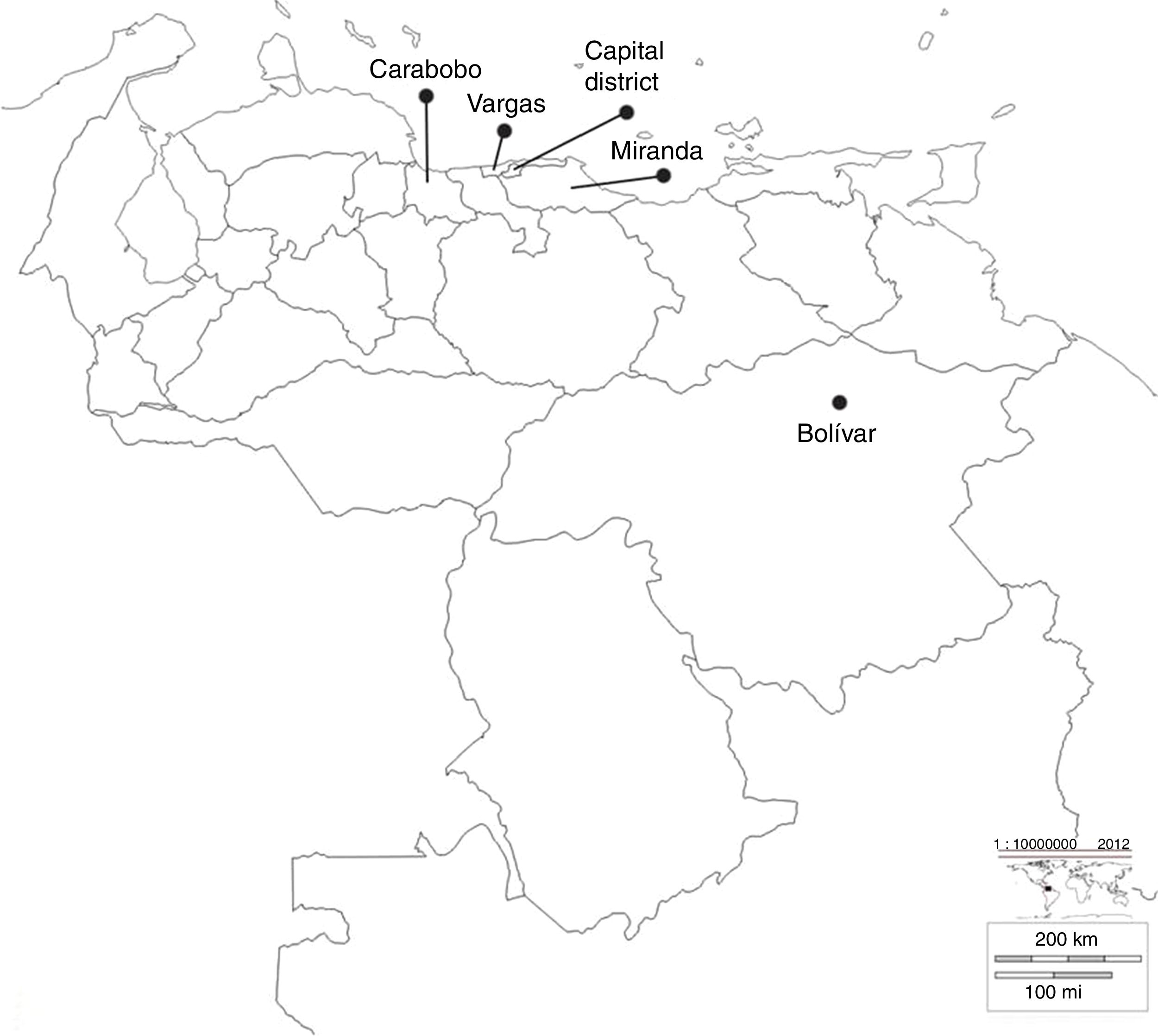
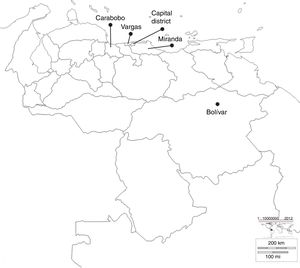
ResultsIsolates studiedThirty KPC-type carbapenemase K pneumoniae isolates were analysed during 2008–2012, from significant outbreaks at different health centres, located in different states of Venezuela, as shown in Fig. 1.
Geographic location of the states where the different health centres included in the study belong. Bolívar: Raúl Leoni Hospital of the Venezuelan Social Security Institute (HRL IVSS); Carabobo: Dr Enrique Tejera Hospital Complex (CHET); Capital District: Santa Ana Maternity (MSA), Latin American Paediatric Cardiology Hospital (HCIL), Dr José María Vargas Hospital (HJMV), Dr Miguel Pérez Carreño Hospital (HMP), J.M. de los Ríos Children's Hospital (HNJMR), La Arboleda Clinic (CA) and Loira Medical Centre (CML); Miranda: La Trinidad Teaching Hospital (CMDLT); Vargas: Pariata Hospital (HP).
97, 93 and 90% of isolates were categorised as resistant to ertapenem, meropenem and imipenem, respectively. The rest of the isolates were categorised with intermediate susceptibility to carbapenems.
Phenotypic characterisation of beta-lactamasesIn 100% of the isolates, the synergy between the boronic acid disc and the evaluated carbapenems was revealed, demonstrating the production of serine protease enzymes. While in the search for metalloenzymes no synergy with EDTA/SMA was observed. 73% of the isolates were positive in the detection of ESBL.
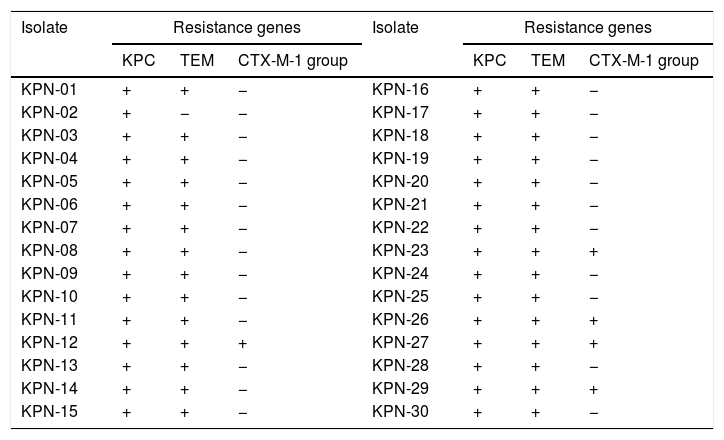
Genotypic characterisation of beta-lactamasesTable 2 shows that in 100% (n=30/30) of the isolates, the gene encoding for KPC-type carbapenemase was detected. The coexistence of blaTEM and blaKPC was detected in 68%, while in 27% the coexistence of blaKPC, blaTEM and blaCTX-M-1 group was detected.
End-point PCR detection of genes encoding for beta-lactamases in isolates of Klebsiella pneumoniae.
| Isolate | Resistance genes | Isolate | Resistance genes | ||||
|---|---|---|---|---|---|---|---|
| KPC | TEM | CTX-M-1 group | KPC | TEM | CTX-M-1 group | ||
| KPN-01 | + | + | − | KPN-16 | + | + | − |
| KPN-02 | + | − | − | KPN-17 | + | + | − |
| KPN-03 | + | + | − | KPN-18 | + | + | − |
| KPN-04 | + | + | − | KPN-19 | + | + | − |
| KPN-05 | + | + | − | KPN-20 | + | + | − |
| KPN-06 | + | + | − | KPN-21 | + | + | − |
| KPN-07 | + | + | − | KPN-22 | + | + | − |
| KPN-08 | + | + | − | KPN-23 | + | + | + |
| KPN-09 | + | + | − | KPN-24 | + | + | − |
| KPN-10 | + | + | − | KPN-25 | + | + | − |
| KPN-11 | + | + | − | KPN-26 | + | + | + |
| KPN-12 | + | + | + | KPN-27 | + | + | + |
| KPN-13 | + | + | − | KPN-28 | + | + | − |
| KPN-14 | + | + | − | KPN-29 | + | + | + |
| KPN-15 | + | + | − | KPN-30 | + | + | − |
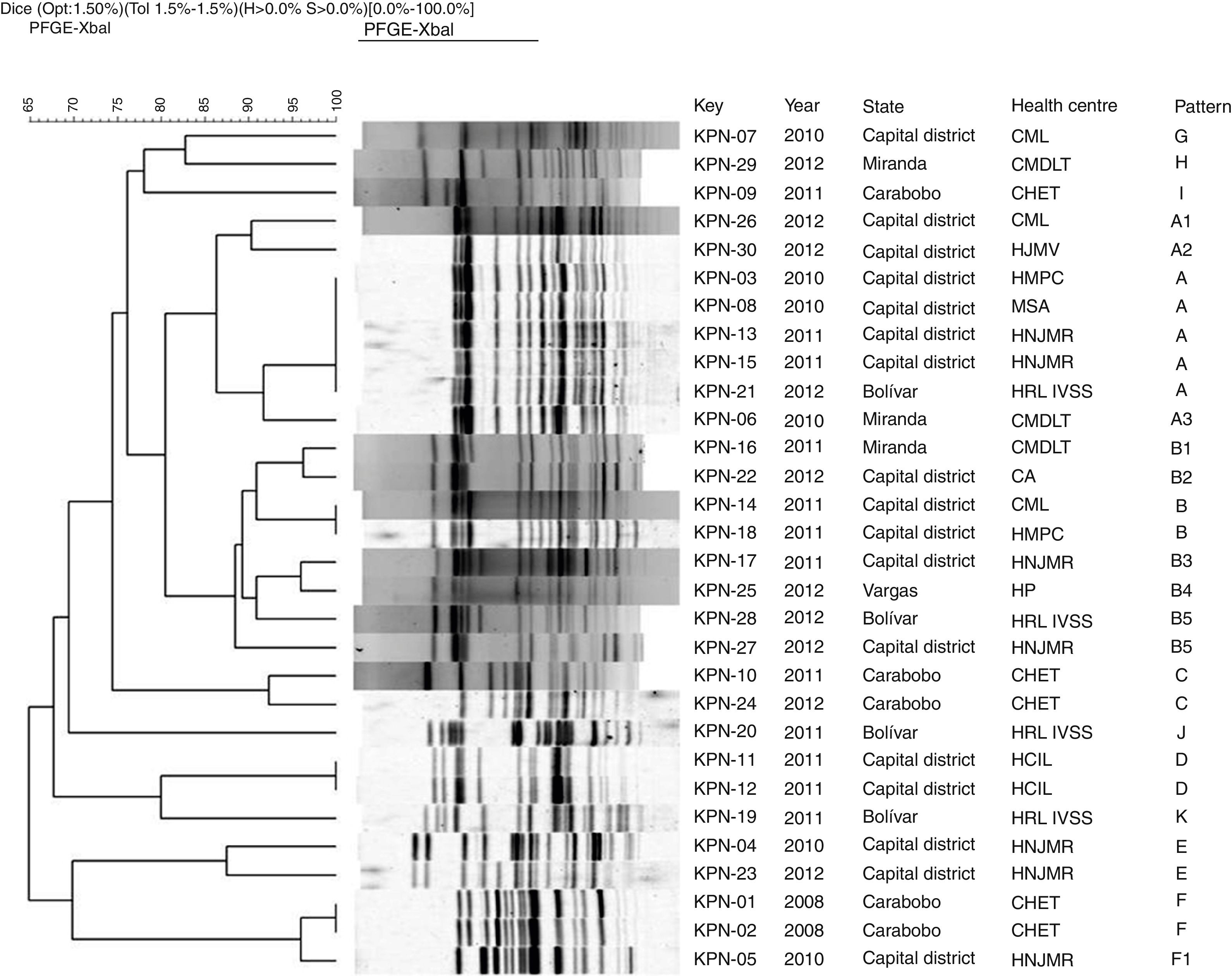
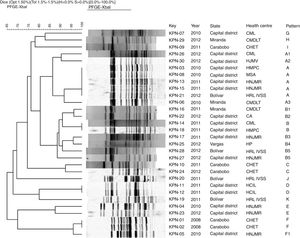
In all the isolates restriction patterns were obtained with the XbaI enzyme (Promega, USA). The number of fragments obtained in the patterns varied between 13 and 19. In the comparative analysis of the genomic DNA patterns obtained by PFE, 11 groupings from the letter A to the letter K were determined (Fig. 2), of which six contained 83% of the isolates studied.
Dendrogram of PFE with XbaI restriction enzyme of 30 KPC Klebsiella pneumoniae isolates from different health centres of Venezuela from 2008 to 2012. UPGMA, Dice tolerance coefficient 1.5%. Optimisation 1.5%.
CA: La Arboleda Clinic; CHET: Dr Enrique Tejera Hospital Complex; CMDLT: La Trinidad Teaching Hospital; CML: Loira Medical Centre; PFE: pulsed-field gel electrophoresis; HCIL: Latin American Paediatric Cardiology Hospital; HJMV: Dr José María Vargas Hospital; HMPC: Dr Miguel Pérez Carreño Hospital; HNJMR: J.M. de los Ríos Children's Hospital; HP: Pariata Hospital; HRL IVSS: Raúl Leoni Hospital of the Venezuelan Social Security Institute; MSA: Santa Ana Maternity Hospital; UPGMA: unweighted pair group method with arithmetic mean.
In group A, five isolates with “indistinguishable” pulsotypes were detected from the Capital District (KPN-03, KPN-08, KPC-13) and Bolívar (KPN-21), as shown in Fig. 2. The rest of the isolates of this group were categorised as “possibly related” (subtypes A1–A3).
In group B, the KPN-14 and KPN-18 isolates from different health centres in the Capital District were categorised as “indistinguishable”, while six isolates from this group were “closely related”. These pulsotypes correspond to isolates derived from health centres located in different states.
Groups C and E each included two isolates recovered in different years which were categorised as “closely related” and corresponded to the health centres CHET and HNJMR, respectively. In groups D and F, “indistinguishable” isolates were detected in the same hospital centre.
The isolates of the G–K patterns showed a percentage of similarity <85%, so they were categorised as “unrelated isolates”, derived between 2010 and 2011 and corresponding to five health centres located in different states with the exception of two isolates recovered in the same health centre, the Raúl Leoni Hospital of the Venezuelan Institute of Social Security (HRL IVSS), in 2011.
When performing the analysis by health centre, it was observed that the isolates of the HRL IVSS, CMDLT, HMPC and CML health centres were “unrelated isolates”. However, intra-hospital clones were detected at the CHET. In the HNJMR, with a percentage of similarity >85%, three groups of closely related isolates were detected from 2010 to 2012. It is important to note that isolates from different years were included in the three groups.
DiscussionK. pneumoniae is an important nosocomial pathogen, whose KPC-type carbapenemase-producing isolates have emerged as a significant problem due to their widespread dissemination worldwide through mobile genetic elements.19
In our study, the majority of the isolates were resistant to the carbapenems evaluated, with the ertapenem disc being the most affected, as well as the results obtained in KPC-type carbapenemase-producing isolates from Norway and Sweden,20 where 100% of the isolates were resistant to ertapenem, while some were categorised with intermediate susceptibility or resistant to the other carbapenems, so it could be considered as the disc that would alert on the possible existence of KPC-type carbapenemase-producing K. pneumoniae isolates. However, the exclusive use of ertapenem as an indicator antibiotic for the suspicion of KPC-type carbapenemase presents problems of specificity.3
The presence of blaKPC in all isolates demonstrated the wide distribution of this gene in different isolates from Venezuela. Other studies conducted in isolation have reported blaKPC in K. pneumoniae from the states of Zulia and Carabobo.7 Cuzon et al. acknowledge that, outside of the United States, KPC-producing K. pneumoniae is reported more frequently, in countries such as France, Israel, Colombia, Brazil, Argentina, China, Greece, Germany, Spain, United Kingdom, Belgium, Croatia, Norway and Poland, among others.1,21
The blaKPC gene has been reported in conjugative plasmids in combination with other resistance genes that encode for the beta-lactamases of the CTX-M-1, TEM and SHV group.7 In the isolates under study, the broad distribution of the gene that encodes for the TEM-type beta-lactamase was evident, in some cases in co-production with the beta-lactamases of the CTX-M-1 group. It is interesting to note that, in a study conducted in Latin America, the TEM gene is not reported in Venezuelan isolates, but it is in high incidence in Guatemala, while blaCTX-M was detected in approximately 50% of isolates.22 The study conducted by Cuzon et al., in different countries, demonstrates the presence of TEM-1 and SHV-11 in isolates from the United States, Greece, Colombia, Brazil and Israel. Likewise, CTX-M-2, CTX-M-12 and CTX-M-15 were reported in these countries with the exception of the United States, Greece and Switzerland, which demonstrates the widespread dissemination of this type of beta-lactamase.21
The results obtained by PFE showed great genetic diversity among the isolates, unlike what was reported in Argentina by Gómez et al., who determined five groups in 69 isolates collected between 2006 and 2010.23 One of them included 91% of the isolates, concluding that the dissemination in Argentina was due to the existence of an epidemic isolate, which was later identified by multilocus sequence typing as ST-258.
However, the results of Cuzon et al. are consistent with the results obtained in our study.21 They reported in 16 isolates from Colombia, Brazil, Israel, the United States, Sweden and Greece heterogeneity among KPC-type carbapenemase-producing isolates when determining nine pulsotypes. In China, Cao et al. demonstrated that the genetic diversity in K. pneumoniae isolates, carriers of multiple resistance genes, including blaKPC, made it possible to make inference about the easy transmission of resistance determinants among bacterial species through mobile genetic elements.24 These results were confirmed by the detection of a high prevalence of integrons and plasmids in their study.
In addition to observing a great variety of patterns, it was determined that some pulsotypes were circulating during the years of study in the 11 health centres of different states of Venezuela, demonstrating the success of this type of isolate to stay over time and disseminate, as has been shown in other studies.25
The presence of similar isolates from different years and health centres could be related to mechanisms such as inter- and intra-hospital dissemination, colonisation of patients, health professionals, etc.; medical equipment or fomites as a vehicle for transmission and the use of broad-spectrum antibiotics.26 Robledo et al. infer that the combination of multiple factors contribute to the dissemination of KPC-type carbapenemase-producing K. pneumoniae isolates. These include the following: failures in the health team to follow the established policies for infection control; increased transit of personnel and medical equipment from different hospitals; the late detection of patients colonised or infected with multidrug-resistant micro-organisms acquired in other institutions; lack of an alert system among laboratories, and/or the inappropriate use of antibiotics.26 Studies conducted in Spain and Greece have inferred about the possible cross-contamination between patients and the medical team, resulting in significant outbreaks of KPC-type carbapenemase-producing K. pneumoniae. However, in countries such as Israel, it has been determined that patients colonised by this type of microorganism play an important role in the dissemination of these isolates.27
In the patterns obtained, genetically-related isolates were observed in the same hospital in different years, which could indicate the permanence of the microorganism in the environment. This factor is favoured by the different properties and characteristics of this bacterium. Some authors indicate that humans can be carriers of K. pneumoniae for many years, with the risk of acquiring infections caused by this microorganism and disseminate it both in hospital environments and in the community, or transmit it among people, as well as among different places in the same hospital and among cities and countries.28
In the analysis of the pulsotypes by years, it was determined that the genetic diversity of the isolates was increasing over time. It is possible that the existence of subtypes is related to the sum of genetic events. The Spanish Society of Infectious Diseases and Clinical Microbiology29 notes that this type of behaviour is to be expected, since at the beginning or in the detection of the first isolates, genetic diversity is likely to be low; however, as genetic changes accumulate in the epidemic or endemic strains, the variety of the patterns obtained by PFE will increase.
Likewise, Sabat et al. state that insertions or deletions of mobile genetic elements, as well as recombination events in genomic DNA, may in some cases result in changes in PFE patterns.30
In this research, the variability of KPC-type carbapenemase-producing isolates was evidenced by PFE (polyclonality) in Venezuela, describing isolates that remained over time and the possible inter- and intra-hospital dissemination in isolates that were found to be genetically related. In this regard, it is necessary to strengthen epidemiological surveillance and the development of activities to prevent and control these types of isolates in health centres in Venezuela.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This study has been possible thanks to the support of the Department of Bacteriology; Epidemiological Diagnostics and Surveillance Management and the Teaching Management of the “Rafael Rangel” National Institute of Hygiene. The collaboration of the personnel of the different sections of bacteriology of the health centres who derived the strains studied. The collaboration of the personnel of the Laboratory of Bacterial Isolation and Identification of the Rafael Rangel National Institute of Hygiene. Dr Omaira Da Mata and Graduate Cirana Rodríguez for their advice on the PFE technique.
Please cite this article as: Cuaical-Ramos NM, Montiel M, Marcano Zamora D. Variabilidad genética de Klebsiella pneumoniae con carbapenemasa tipo KPC proveniente de diferentes estados de Venezuela. Enferm Infecc Microbiol Clin. 2019;37:76–81.