The majority of malaria cases diagnosed in Europe in the last few years have occurred in people living in non-endemic areas travelling back to their home country to visit friends and relatives (VFRs). Children account for 15–20% of imported malaria, with known higher risk of severe disease.
Material and methodsA retrospective multicentre study was conducted in 24 hospitals in Madrid (Spain) including patients under 16 years diagnosed with malaria (2007–2013).
ResultsA total of 149 episodes in 147 children were reported. Plasmodium falciparum was the species most commonly isolated. Twenty-five patients developed severe malaria and there was one death related to malaria. VFR accounted for 45.8% of our children. Only 17 VFRs had received prophylaxis, and 4 of them taken appropriately. They presented more frequently with fever (98% vs. 69%), a longer time with fever (55 vs. 26%), delay in diagnosis of more than three days (62 vs. 37%), and more thrombocytopenia (65 vs. 33%) than non-VFRs, and with significant differences (p<0.05).
ConclusionsVFRs represent a large proportion of imported malaria cases in our study. They seldom took adequate prophylaxis, and delayed the visit to the physician, increasing the length of fever and subsequent delaying in diagnosis. Appropriate preventive measures, such as education and pre-travel advices should be taken in this population.
En los últimos años la mayoría de los casos de malaria en Europa se han producido en personas asentadas en zonas no endémicas que viajan a su país de origen para visitar a amigos y familiares (VFR). Los niños representan el 15-20% de la malaria importada, con el conocido alto riesgo de enfermedad grave.
Material y métodosEstudio multicéntrico retrospectivo en 24 hospitales en Madrid, que incluyó pacientes menores de 16años con diagnóstico de malaria (2007-2013).
ResultadosSe registraron 149 episodios en 147 niños. Plasmodium falciparum fue la especie más frecuentemente aislada. Veinticinco niños padecieron paludismo grave y hubo una muerte relacionada con la malaria. Los pacientes VFR representaron el 45,8% de nuestros niños estudiados. Solo 17 de los VFR habían recibido profilaxis y en solo 4 casos la tomaron apropiadamente. Estos pacientes presentaron con más frecuencia fiebre (98% vs 69%), retraso en el diagnóstico más de 3 días (62 vs 37%) y trombocitopenia (65 vs 33%).
ConclusionesLos niños VFR representaron una gran proporción de casos de paludismo importado en nuestro estudio. Rara vez tomaron la profilaxis de forma adecuada. Además estos niños presentaron un mayor retraso en la consulta al médico tras la aparición de síntomas, con el subsiguiente retraso en el diagnóstico. Es necesario tomar las medidas preventivas adecuadas, como la educación o el consejo pre-viaje, en esta población.
Great efforts have been developed in the last years to fight against malaria worldwide. Although there has been a reduction in the incidence in the past years, especially in some regions of Africa and the region of Americas, there were still an estimated 214 million cases of malaria in 2015 and 438,000 deaths worldwide. Ninety percent of all malaria deaths occur in sub-Saharan Africa, and 70% in children under five years-old.1
Even though the global burden of malaria is largely carried by endemic areas, globalization has driven to a pronounced rise in the incidence of imported malaria in non-endemic regions.2 Imported malaria is defined as an infection acquired in a malaria-endemic area but diagnosed in a non-endemic area after development of clinical symptoms.2 Children represented around 15–20% of total cases of imported malaria cases, and it was estimated that around 5–10% of them fulfilled criteria for severe malaria according to WHO definition.2,3
They are known to have a higher risk of developing severe disease since they are more likely to be non-immune to malaria.2
Over the past years the majority of malaria cases in Europe have occurred in adults and children settled in non-endemic countries but have travelled to their home country on holidays to visit friends and relatives (VFRs).4,5 Nevertheless in recent years there have been as well changes in immigration patterns, particularly countries accepting immigrants, refugees and adopted children from endemic areas. These groups usually had partial immunity and therefore could be more likely to suffer non-severe disease.5,6
Malaria was declared eradicated in Spain in 1964, and since 1995 a mandatory weekly notification is established. The last temporary bulletin, reported 545 accumulated cases in 2014, 185 in Madrid.7,8
The aim of this study was to describe the epidemiological and clinical features of children diagnosed in Madrid in recent years, as well as their outcomes.
Patients and methodsA retrospective study was performed in 24 hospitals (five of them tertiary) from the Public Health System in the Community of Madrid (CAM), Spain. All children younger than 16 years diagnosed with malaria between January 2007 and January 2013 were included. This study was approved by the Ethics Committee.
Main characteristics of the study participants, including epidemiological data (country of origin, travel history, antimalarial prophylaxis, underlying conditions or concurrent infections, previous history of malaria), clinical features (symptoms, complications, admissions, management and outcome), and laboratory and microbiological studies, were obtained from medical records.
The burden of malaria in children was calculated according to the estimated number of children living in CAM from 2007 to 2013.
A patient was considered VFR, when living in a non-endemic country and travelling to visit their friends or relatives in their parent's country of origin, as previously described in the literature.2 The non-VFR group included children born in endemic areas who came to Spain, including immigrants, adopted children or travellers from endemic areas arrived to Spain.
Diagnosis of malaria was done in the basis of parasite visualization through microscopic examination of thick and thin blood smears and/or rapid detection tests (RDTs) or molecular techniques detection. The level of parasitaemia was expressed as the percentage of parasitized erythrocytes. Molecular techniques based on DNA amplification (multiplex polymerase chain reaction [PCR]) for the four species of Plasmodium were conducted at the Parasitology Department of the National Microbiology Centre (Madrid). RDTs used varied according to participating Hospitals, including: BinaxNOW Malaria test, (Binax Inc, Scarborough, ON), Immunoquick Malaria Falciparum, (BioSynex Inc., France), Biotech, (BioSynex Inc., France), Core Malaria Pan/Pf (Core Diagnostics Inc., United Kingdom) and SD Bioline Malaria Ag Pf/Pan (Standard Diagnostics Inc, Korea), detecting all of them panmalaric and falciparum antigen. Vivax or other species could be detected depending on the RDT.
Laboratory values: anaemia was defined as haemoglobin (Hb) concentration values below the normal range for age, and thrombocytopenia as platelets less than 150,000/μl. Hypoglycaemia was defined as blood glucose less than 45mg/dL.
Severe falciparum malaria was defined according to the World Health Organization criteria.9
Antimalarial therapies were collected in database, as well as additional support measures, including transfer to Paediatric Intensive Care Units, intravenous treatment or additional support measures if needed.
Haemodynamic support item included the use of colloid/crystalloid fluids, or vasoactive drugs. Respiratory support included the use of oxygen, or assisted ventilation.
Data were entered into Microsoft Excel database (Office for Windows XP, 2003 and 2010) and analyzed using SPSS software, version 15.0 (SPSS). Categorical variables were expressed as frequencies and percentages; numerical variables were presented as means and standard deviation (SD), or medians and interquartile ranges (IQR), depending on distribution. Categorical variables were compared using Chi-square, linear Chi-square tendency or Fisher test when appropriate.
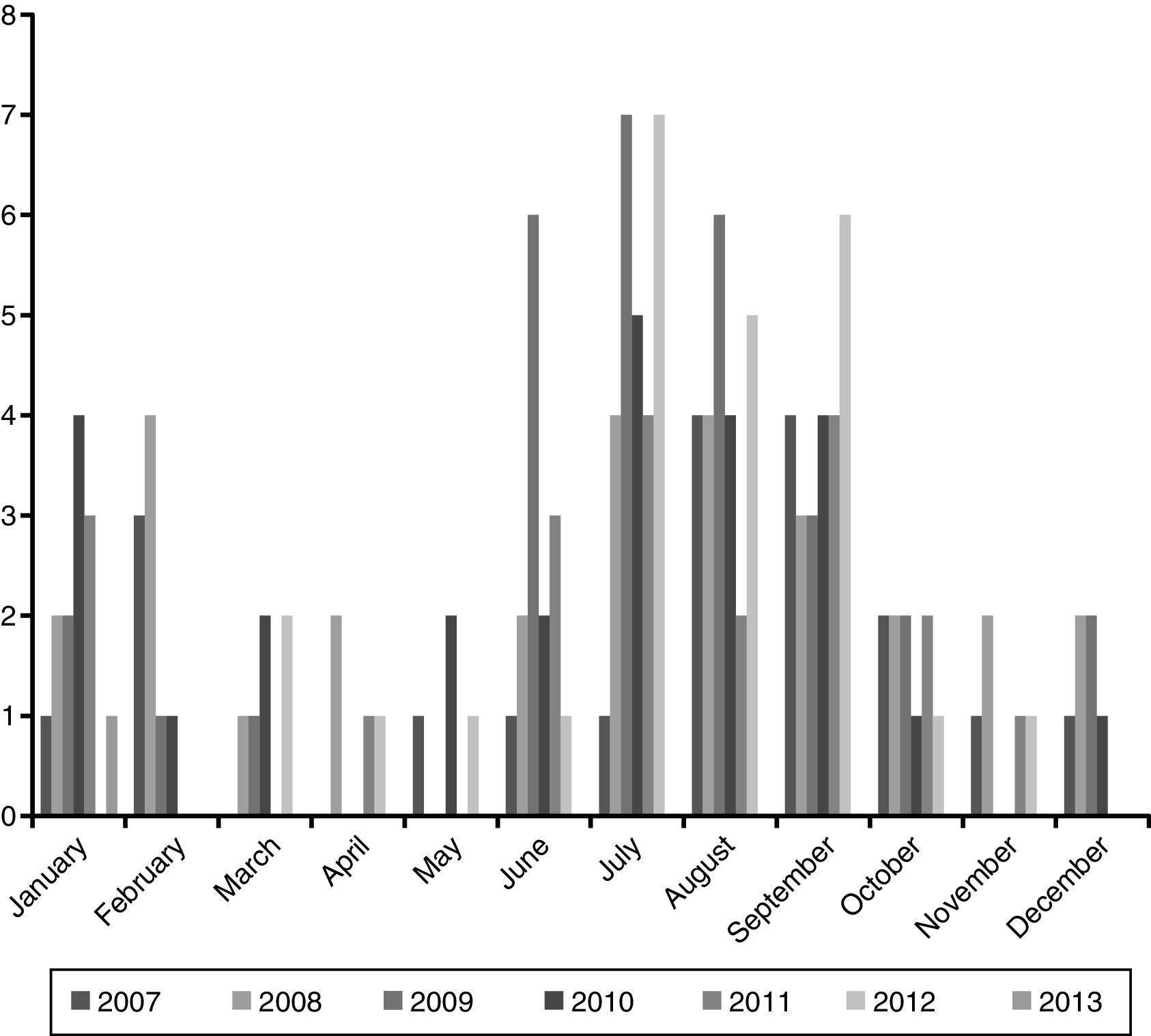
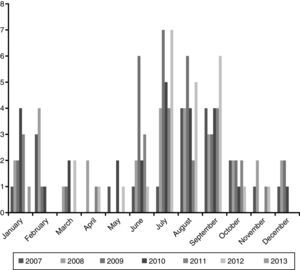
ResultsEpidemiological characteristicsDuring the study period 149 episodes of malaria were reported in 147 paediatric patients (49% males). The estimated burden of malaria in children in CAM for this period was 1.68/100,000 per year. The median age at diagnosis was 72 months (IQR 35–119), and 39% were younger than 5 years old. Every year most cases were reported during summer (June–September), with a small peak between January and February. The trend along the years of the study remained stable (Fig. 1).
Patients were commonly born in foreign countries (61%), especially Sub-Saharan Africa (98%), Equatorial Guinean patients accounting for 45% overall. Only 3 cases were reported from Asia, corresponding to three adoptees from India. No cases from the region of Americas or Oceania were registered during the period of the study, Sixty-six patients (45.8%) fulfilled the criteria for VFRs, most of them travelling to Sub-Saharan Africa. Most of non-VFRs were immigrants; only 4 of them (4/81, 4.9%) were adopted and 2 of them (2/81, 2.5%) refugees.
Eighteen patients (18/139, 12.9%) had received prophylaxis, and in 4 of them it was taken appropriately.
Seventy-five children (51%) had had prior episodes of malaria. Five patients (3.4%) had HIV infection, three patients (2%) had sickle cell disease and one patient (0.6%) was affected by Burkitt lymphoma.
Clinical featuresThe main symptom, present in 82% of patients, was fever. The median days of fever prior to diagnosis of malaria were 2 days (IQR 1–4). The remaining 27 afebrile patients were mainly immigrants or adopted children, being only 2 of them VFRs.
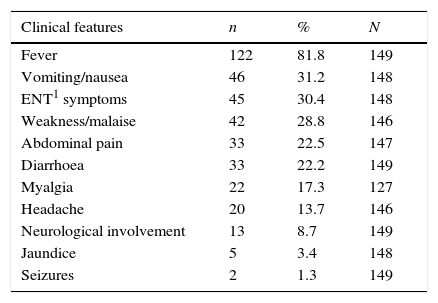
Most of the patients were in a good general condition. The most common signs found were hepatomegaly (39%) and enlarged spleen (37%). Neurological involvement with impaired consciousness was reported in 13 children (8.7%). Table 1 shows initial clinical characteristics and physical examination.
Initial characteristics among 149 episodes of imported malaria in children in CAM, 2007–2013.
| Clinical features | n | % | N |
|---|---|---|---|
| Fever | 122 | 81.8 | 149 |
| Vomiting/nausea | 46 | 31.2 | 148 |
| ENT1 symptoms | 45 | 30.4 | 148 |
| Weakness/malaise | 42 | 28.8 | 146 |
| Abdominal pain | 33 | 22.5 | 147 |
| Diarrhoea | 33 | 22.2 | 149 |
| Myalgia | 22 | 17.3 | 127 |
| Headache | 20 | 13.7 | 146 |
| Neurological involvement | 13 | 8.7 | 149 |
| Jaundice | 5 | 3.4 | 148 |
| Seizures | 2 | 1.3 | 149 |
| Physical examination | N | % | N |
|---|---|---|---|
| General condition | 149 | ||
| Good | 119 | 80 | |
| Acceptable | 28 | 18.8 | |
| Bad | 2 | 1.3 | |
| Hepatomegaly | 58 | 39 | |
| Splenomegaly | 55 | 37 | |
| Hepatosplenomegaly | 39 | 26.2 | |
| Pallor | 33 | 22.1 | |
| Impaired consciousness | 13 | 8.8 | |
| Jaundice | 12 | 8.1 | |
| Work on breathing | 4 | 2.7 | |
| Initial laboratory and microbiology results | N | % | N |
|---|---|---|---|
| Anaemia | 107 | 71.8 | 149 |
| Thrombocytopenia | 69 | 46.3 | 149 |
| Microbiology testsa | |||
| Thick blood film | 103 | 83.7 | 123 |
| Thin blood film | 94 | 79 | 119 |
| RDT | 92 | 90.2 | 102 |
| PCR | 56 | 100 | 56 |
| Initial parasitaemia | |||
| >2% | 38 | 30 | 124 |
| >5% | 18 | 14 | 124 |
a Table shows results from POSITIVE tests among total performed.
One hundred and seven patients (71.8%) had Hb values below the normal range for their age. Sixty-four patients (42.9%) had Hb less than 10g/dL, and only three (2%) had less than 5g/dL. Thrombocytopenia was found in 69 of them (46.3%).
No relevant abnormalities were found in general in liver and renal function. Hypoglycaemia was found in one patient, due to quinine infusion. Nine out of 117 patients (7.7%) had haemoglobinuria.
Lumbar puncture was performed in six patients, four of them with impaired consciousness; none of them had significant abnormalities.
MicrobiologyThick blood film was performed in 123 samples, whereas thin blood film was performed in 119, being positive in 83.7% and 79% respectively. When both of them were done it increased the positivity rate to 91.1%. Positive results for RDTs were observed in 92/102 episodes (90.2%). Twelve (8%) were diagnosed based only on PCR (6 of them RDT negative, were immigrant children, two of them had fever and only one of them had dehydration signs, the rest remaining asymptomatic).
Initial median parasitaemia measured was 0.25% (IQR 0.1–2.6). Thirty-eight patients (38/124, 30%) had initial parasitaemia higher than 2% and 18/124 (14%) higher than 5%.
Plasmodium falciparum was the species most commonly isolated, in 135 episodes (90.6%); Plasmodium ovale was isolated in 10 samples (6.7%), and Plasmodium malariae in 3 samples (2%). In 8 cases (5.4%) identification of Plasmodium species was not possible (Plasmodium spp.). Nine mixed parasitaemia (6%) were detected.
Management and outcomeOf the 149 episodes of malaria, 128 (86%) were admitted in hospitals, with a median length of stay of 5 days (IQR 3–7). Twenty-five cases (17%) met at least one criteria of severe falciparum malaria according to WHO guidelines.
Fourteen (9%) cases were transferred to Paediatric Intensive Care Units (PICUs). Of them, ten fulfilled criteria for severe malaria.
Intravenous (IV) treatment was administered in 19 (12.7%) children. The most common combination was Quinine plus Clindamycin, although in two of them it was switched to Artesunate (in tertiary hospitals). The median length on IV treatment was 2.5 days (IQR 2–5). All patients received oral therapy (either initially or sequenced after first IV doses). The most common drug administered orally was Atovaquone-Proguanil, in 96 patients (65%), with a median duration of 3 days (IQR 3–7). Six children (4%) received Primaquine (due to mixed parasitaemia).
Adverse effects were noticed in five patients (3.3%), being gastrointestinal disorder the most common, and none of them severe.
Thirteen children (8.7%) needed blood transfusion. Antibiotherapy was administered in 25 patients (18.7%), being Cefotaxime the most common drug used (13/25, 52%). No bacteraemia was reported in data collected. Respiratory support was provided in 4 children (2.7%), only one requiring invasive mechanical ventilation. Haemodynamic support was needed in nine patients (6%), with crystalloid fluids, two of them requiring inotropic drugs.
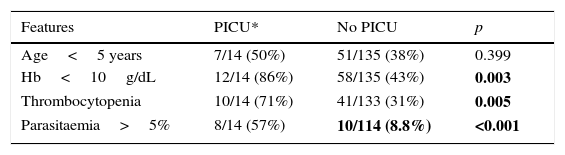
When analysing children admitted in PICU we found more related thrombocytopenia, higher parasitaemia, and Hb less than 10g/dL with statistically significant differences (Table 2).
Comparison between children with paludism in CAM (2007–2013) admitted and non-admitted in PICU.a
| Features | PICU* | No PICU | p |
|---|---|---|---|
| Age<5 years | 7/14 (50%) | 51/135 (38%) | 0.399 |
| Hb<10g/dL | 12/14 (86%) | 58/135 (43%) | 0.003 |
| Thrombocytopenia | 10/14 (71%) | 41/133 (31%) | 0.005 |
| Parasitaemia>5% | 8/14 (57%) | 10/114 (8.8%) | <0.001 |
Regarding final outcome, 140 (94%) out of the 149 episodes fully recovered. Two children relapsed (1.3%), one corresponding to oral intolerance to antimalarial drugs and the other one to mixed P. falciparum and P. ovale (parasitaemia). Five patients were lost during the follow-up.
Two patients died. One of the deceases was reported in a 22 months old patient, born in Spain, VFR travelling to Nigeria with no underlying condition known. Initially he presented with fever and gastrointestinal disorders. P. falciparum was identified in blood smear (initial median parasitaemia 0.1%). He had a sudden worsening, with severe anaemia (Hb 3.1g/dL), metabolic acidosis and impaired liver function that led to cardiorespiratory arrest after 2 days of oral treatment. A patient affected with Burkitt lymphoma died by a non-malaria related cause.
Characteristics of population VFR and comparison with non-VFR patientsSixty six children (45%) were VFRs. Most of them travelled to Sub-Saharan Africa (Equatorial Guinea accounting the 72% of overall VFRs) with a median length of stay of 60 days (IQR 29–118.5). Only 17 VFRs had documented prophylaxis, being mefloquine the most common therapy, and only four of them had adequate adherence.
Of the 28 VFRs with prior episode of malaria, prophylaxis was registered in four of them (14.3%), and none of them was taken appropriately. Fever was the most common clinical feature, present in 98% of them (64/66).
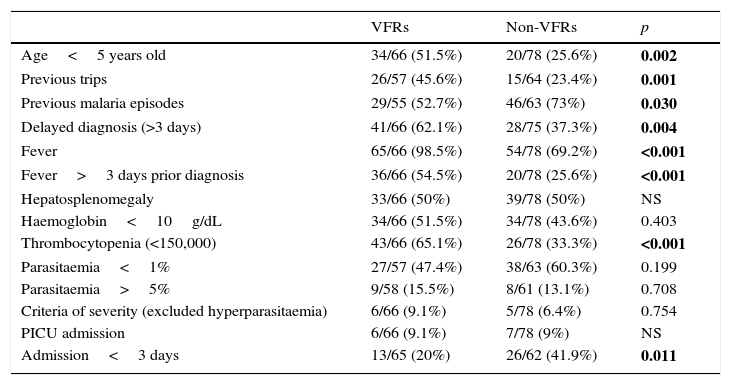
When compared with non VFRs we found significant differences in the presence of fever (98 vs. 69%, p<0.001), length of fever more than three days (55 vs. 26%, p<0.001) and delaying in diagnosis more than three days (62 vs. 37%, p=0.004).
They had more frequently thrombocytopenia (65 vs. 33%, p<0.001), but there were no differences regarding anaemia. No differences were found in the percentage of parasitaemia between groups. Results are shown in Table 3.
Comparison between VFRsa and non-VFRs paediatric patients during the period of study.
| VFRs | Non-VFRs | p | |
|---|---|---|---|
| Age<5 years old | 34/66 (51.5%) | 20/78 (25.6%) | 0.002 |
| Previous trips | 26/57 (45.6%) | 15/64 (23.4%) | 0.001 |
| Previous malaria episodes | 29/55 (52.7%) | 46/63 (73%) | 0.030 |
| Delayed diagnosis (>3 days) | 41/66 (62.1%) | 28/75 (37.3%) | 0.004 |
| Fever | 65/66 (98.5%) | 54/78 (69.2%) | <0.001 |
| Fever>3 days prior diagnosis | 36/66 (54.5%) | 20/78 (25.6%) | <0.001 |
| Hepatosplenomegaly | 33/66 (50%) | 39/78 (50%) | NS |
| Haemoglobin<10g/dL | 34/66 (51.5%) | 34/78 (43.6%) | 0.403 |
| Thrombocytopenia (<150,000) | 43/66 (65.1%) | 26/78 (33.3%) | <0.001 |
| Parasitaemia<1% | 27/57 (47.4%) | 38/63 (60.3%) | 0.199 |
| Parasitaemia>5% | 9/58 (15.5%) | 8/61 (13.1%) | 0.708 |
| Criteria of severity (excluded hyperparasitaemia) | 6/66 (9.1%) | 5/78 (6.4%) | 0.754 |
| PICU admission | 6/66 (9.1%) | 7/78 (9%) | NS |
| Admission<3 days | 13/65 (20%) | 26/62 (41.9%) | 0.011 |
This is to our knowledge one of the largest series of imported malaria in children in our country. There are few studies of paediatric malaria in developed countries to date.
The high proportion of children less than five years observed is concerning, being this group at special risk of developing severe malaria. We observed also that many of them use to travel to endemic-areas without the appropriate prophylaxis measures. This feature has been observed also in other imported malaria series in Western Europe such as Ladhani et al.10
Seventy-five children had had other malaria episodes prior the current, 42.4% out of the overall VFRs, and 56.8% of immigrant/adopted children. Of the total amount of VFRs with previous malaria only four of them had received prophylaxis, and none of them was taken appropriately. This high numbers should encourage physicians to educate and follow-up those children susceptible to travel to endemic areas, and prescribe them adequate prophylaxis when applicable.5
As described in other European series the majority of children were from sub-Saharan Africa settings. In our study almost 45% of the patients were from Equatorial Guinea. It is likely due to have been a former Spanish colony.11 Interestingly we have not observed imported malaria in children from the Americas during the study period, being Spain still commonly selected by immigrants from those areas (in 2009 the 30% of the total amount of immigrants were from Ecuador, Colombia, Peru and Bolivia).11 It subscribes, nevertheless, to the last WHO malaria report, showing a decrease in number of estimated cases of 80% in the region of the Americas.1
Paediatricians must still be aware of the unspecific features of malaria in early stages. Fever, although frequent, is not always present (and, in our series, especially in non-VFRs), and respiratory and gastrointestinal symptoms are common.10–13 In addition, it is described that most children with severe malaria have neurologic involvement rather than respiratory distress or severe anaemia.14,15
It is remarkable the absence of Plasmodium vivax isolation. Interestingly there were not children from the Americas region, where P. vivax is more common. P. vivax is a well-defined cause of malaria in sub-Saharan Africa; however, it is less common in Western Africa, including Equatorial Guinea.1 That may explain the results in our series.16,17
Special attention deserves the group of patients VFRs. Over the past years there is a significant increase of malaria cases in Europe people settled in non-endemic countries but travelling to their home country on holidays to visit friends and relatives (VFRs).2,18 This group is less likely to seek pretravel advice or take prophylaxis, and more likely to travel to rural-endemic areas for longer periods. It is more common as well the delay in seeking medical help when returning. Although in our series we did not observe more risk of severe malaria, they used to have fever more frequently, longer fever and more delayed diagnosis than those immigrants or adopted children. Prevention measures are especially mandatory in this group of patients, including chemoprophylaxis or vector control measures.5,19
The management in children malaria has been a challenge for clinicians, since there have not been many studies until recently about safeness and efficacy of drugs in them, and they are off license in some countries for paediatric population.
The 2005 SEAQUAMAT study and subsequent 2010 AQUAMAT, this one performed in 5425 African children, revealed a significant reduction of mortality and clinical complications in patients receiving IV artesunate compared with quinine in severe falciparum malaria.20,21 These trials, along with other similar22 have led to a change in WHO guidelines,23 and therefore the recommendations in other developed areas such as Australia, Canada or USA.24–28 Nonetheless, to date this drug is not licensed for use in some countries,29 hence not always available in their hospitals; it leaves the combination quinine plus doxy/clinda remaining an acceptable option.24,27,28
The current recommendations in Spain30 agree with the use of parenteral artesunate as the treatment of choice in these patients. However, artemisinin derivatives were not available at least widely during most of the years of the study; subsequently many of our children were treated with quinine, needing more days of admission. Given the recommendations worldwide we encourage the use of artesunate when available.
In uncomplicated falciparum malaria it is recommended the use of atovaquone-proguanil, and equally, if available, artemisinin derivatives as first choice.24–30 Most of our patients were managed with atovaquone-proguanil, with adequate oral tolerance and no remarkable side effects.
One of the limitations of our study was the retrospective design, and heterogeneous management in some cases, given the different hospitals, and different population of each area.
ConclusionsThe characteristics of children with imported malaria in Madrid are similar to those previously described in Western Europe. Epidemiological changes in worldwide malaria infection are to be noticed, as observed in our study, with no episodes of malaria observed in children coming from the region of Americas.
Over the past years there are concerns based on the reports of malaria cases in Europe from children VFRs. We observed that these children delay the visit to the physician, dilating the length of fever, and subsequent delaying in diagnosis.
The low rates of appropriate prophylaxis are concerning as well. Further studies to assess the barriers for pre-travel advices, appropriate prophylaxis and adequate follow up after returning are mandatory.
FundingThe authors acknowledge no financial support.
Conflict of interestsThe authors declare that they have no conflict of interest.
- -
Hospital Universitario 12 de Octubre: M García Ros.
- -
Hospital Universitario La Paz-Carlos III: I De Augusto, C Calvo, M García López Hortelano.
- -
Hospital General Universitario Gregorio Marañón: MC Suarez.
- -
Hospital Universitario Fundación Jiménez Díaz: AB Jiménez.
- -
Hospital General de Móstoles: S Valderrama.
- -
Hospital Severo Ochoa Leganés: I Aguado.
- -
Hospital Príncipe de Asturias: E Pérez.
- -
Hospital Ramón y Cajal: C. Vázquez.
- -
Hospital Universitario de Getafe: B Soto, LM Prieto, S Guillén, M Esquivias.
- -
Hospital Clínico San Carlos: A Vieco. JT Ramos.
- -
Hospital Universitario de Fuenlabrada: C Grasa, I. Rivero.
- -
Hospital Infantil Universitario Niño Jesús: M Sierra.
- -
Hospital Universitario Fundación Alcorcón: L Morán.
- -
Hospital Universitario Infanta Leonor: B Agúndez.
- -
Hospital Universitario Infanta Cristina: J Jensen.
- -
Hospital del Henares: I. Maté.
- -
Hospital Universitario de Torrejón: K Badillo.
- -
Hospital Universitario Infanta Elena: FJ Sanz.
- -
Hospital Universitario Puerta de Hierro Majadahonda: MT García.
- -
Hospital Universitario Infanta Sofía: A Tagarro.
- -
Hospital del Tajo: M Orio.
- -
Hospital Universitario del Sureste: M Llorente.
- -
Hospital Central de la Defensa Gómez Ulla: L Escosa.