Catheter-related bloodstream infection (C-RBSI) can sometimes be managed without catheter removal by combining systemic therapy with catheter lock therapy. Most antiseptic lock solutions are made up of ethanol combined with an anticoagulant. However, data regarding the anti-biofilm activity of ethanol combined with enoxaparin are scarce. We aimed to assess the efficacy of ethanol at different concentrations combined with enoxaparin 60IU as a lock solution for eradication of the biofilm of different microorganisms.
MethodsUsing a static 96-well plate in vitro model, we tested 30%, 35%, and 40% ethanol alone and combined with 60IU of enoxaparin against 24-h-old biofilm from the following microorganisms: Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, and Candida albicans. Time of exposure was assessed during a 2-h and 24-h regimen. We analysed the percentage reduction in metabolic activity using the XTT assay. We considered therapy to be successful when metabolic activity was reduced by >90%.
ResultsIn the 2-h regimen, the therapy was successful against all microorganisms at 35% and 40% ethanol without enoxaparin (p<0.001). In the 24-h regimen, the therapy was successful against all microorganisms at all ethanol solutions without enoxaparin (p<0.001). When ethanol was combined with enoxaparin, the therapy was only successful in the 24-h regimen in biofilms of S. epidermidis, C. albicans and E. coli at all concentrations of ethanol assessed.
ConclusionsOur in vitro model demonstrated that when ethanol is combined with enoxaparin in a lock solution, it negatively affects ethanol anti-biofilm activity after both short and long exposures.
La bacteriemia relacionada con el catéter (BRC) puede ser manejada sin la retirada del catéter mediante la combinación de terapia sistémica y terapia de sellado de catéter. Las soluciones de sellado con antisépticos más utilizadas están compuestas de etanol combinado con anticoagulante. Sin embargo, los datos sobre la actividad anti-biopelícula del etanol combinado con enoxaparina son escasos. Nuestro objetivo fue evaluar la eficacia del etanol a diferentes concentraciones combinado con enoxaparina 60UI como solución de sellado para la erradicación de la biopelícula de diferentes microorganismos.
MétodosMediante un modelo in vitro estático en placa de 96 pocillos, testamos etanol al 30, 35 y 40% solo y combinado con 60UI de enoxaparina frente a una biopelícula de 24h de los siguientes microorganismos: Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli y Candida albicans. El tiempo de exposición se evaluó durante un régimen de 2 y 24h. Se analizó el porcentaje de reducción de la actividad metabólica mediante el ensayo XTT. Consideramos éxito de la terapia cuando la actividad metabólica se redujo >90%.
ResultadosEn el régimen de 2h, la terapia fue exitosa contra todos los microorganismos a concentraciones de etanol del 35 y 40% sin enoxaparina (p<0,001). En el régimen de 24h, la terapia fue exitosa contra todos los microorganismos en todas las soluciones de etanol sin enoxaparina (p<0,001). Cuando se combinó etanol con enoxaparina, la terapia solo tuvo éxito en el régimen de 24h en todas las concentraciones de etanol evaluadas frente a biopelículas de S. epidermidis, C. albicans y E. coli.
ConclusionesNuestro modelo in vitro demostró que la adición de enoxaparina a etanol en solución de sellado afecta negativamente a la actividad anti-biopelícula del etanol tanto tras exposiciones cortas como largas.
Catheter-related bloodstream infection (C-RBSI) is one of the most common nosocomial infections. Approximately 1–2 episodes per 1,000 catheter days have been recorded in intensive care units (ICU), and the mortality rate has been reported to rise to 25%.1–3
The distribution of causative microorganisms is as follows: Gram-positive cocci, 70% (coagulase-negative staphylococci, Staphylococcus aureus, enterococci); Gram-negative bacilli, 20% (Escherichia coli, Klebsiella pneumoniae); and yeasts, 10% (Candida spp.).2,4,5 The ability of bacteria and yeasts to cause C-RBSI depends on their capacity to form biofilms on the catheter surface, and their adherence to medical devices promotes persistent infection and treatment failure.6,7
Guidelines recommend removing the catheter when C-RBSI is suspected. However, when the infection has to be managed with the catheter in place, combining systemic antimicrobial treatment with antibiotic lock therapy (ALT) is also recommended.4,8–12 ALT is based on the instillation of approximately 2ml of a high concentration of antimicrobials (MIC ×100–×1,000) for 2–24h.10 However, the properties of biofilm and the increasing frequency of multidrug-resistant strains are leading ALTs to fail.13 Alternatively, antiseptic lock solutions have been proposed as a novel alternative to ALTs, as no resistance to antiseptic has been reported.14,15
Ethanol is one of the most commonly used antiseptics in the clinical setting. It is administered in combination with an anticoagulant to prevent catheter occlusion and cracking after more than 2h of antiseptic lock therapy.16 In a recent in vitro study, ethanol was shown not to affect the activity of enoxaparin.17 However, to the best of our knowledge, the efficacy of combining ethanol with enoxaparin as a catheter lock solution has not been assessed against microbial biofilms in a 96-well microplate.
Our objective was to test the efficacy of ethanol at 30%, 35%, and 40% alone and in combination with enoxaparin 60IU as a lock solution for eradication of pre-formed biofilms of various microorganisms.
Materials and methodsWe ran a static in vitro model in a 96-well polystyrene plate in which ethanol-based lock solutions were tested against 24-h-old biofilms of the following strains: S. aureus ATCC25923, Staphylococcus epidermidis (clinical strain), Enterococcus faecalis ATCC33186, E. coli ATCC25922, and Candida albicans ATCC14058.
Laboratory procedureA loopful of 24-h-old culture of the following strains was inoculated into 20ml of their corresponding liquid media: staphylococci in Tryptic Soy Broth (TSB), E. faecalis in TSB enriched with 1% glucose, C. albicans in Roswell Park Memorial Institute (RPMI), and E. coli in Luria-Bertani Broth (LB).18–20 Microbial suspensions were cultured overnight at 30°C under orbital shaking. Biofilms were formed as described elsewhere.21 Briefly, inoculums were washed 3 times with phosphate buffered saline (PBS) and adjusted to an optical density of 0.5McFarland, or 0.35McFarland in the case of C. albicans. From this suspension, 100μl was added to each well, and plates were cultured at 37°C for 24h. Each strain was tested 12 times using a positive control treated with medium and a negative control treated without microorganisms. After incubation, plates were washed 3 times with PBS and 120-μl ethanol-based solutions mixed with 120μl of medium were added to each well except in positive control wells, where only 120μl of medium was added. Plates were incubated again for 2 and 24h at 37°C, before being washed 3 times with PBS and dried completely. Then, 100μl of XTT-menadione (10ml 1mg/ml–40μl 1.72mg/ml), which was prepared immediately before the experiment, was added to each well, and the plates were incubated in darkness at 37°C for 2h. Absorbance was measured at 492nm in a spectrophotometer (Biochrom EZ Read 400).
Preparation of ethanol-based solutionsSolutions were prepared immediately before each experiment as follows: 30%, 35%, and 40% ethanol alone and with 60IU of enoxaparin (Clexane® 40mg, 4000IU, enoxaparina sódica, Sanofi-Aventis, SA.A, Barcelona, Spain) (as is the standard used in our institution for catheter lock). All ethanol concentrations are expressed as % (v/v) in distilled water.
Data analysisWe assessed the percentage reduction in metabolic activity by comparing absorbance at 490nm in the positive control wells and in the treated wells. We considered therapy to be successful when metabolic activity was reduced by >90%.
The qualitative variables appear with their frequency distribution. The quantitative variables are summarized as the median (IQR). Continuous variables were compared using the t test; non-normally distributed variables were compared using the Kruskal–Wallis test. The differences between groups were compared using the Mann–Whitney test with a Bonferroni correction. All statistical tests were 2-tailed. Statistical significance was set at p<0.05 for all the tests. Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA).
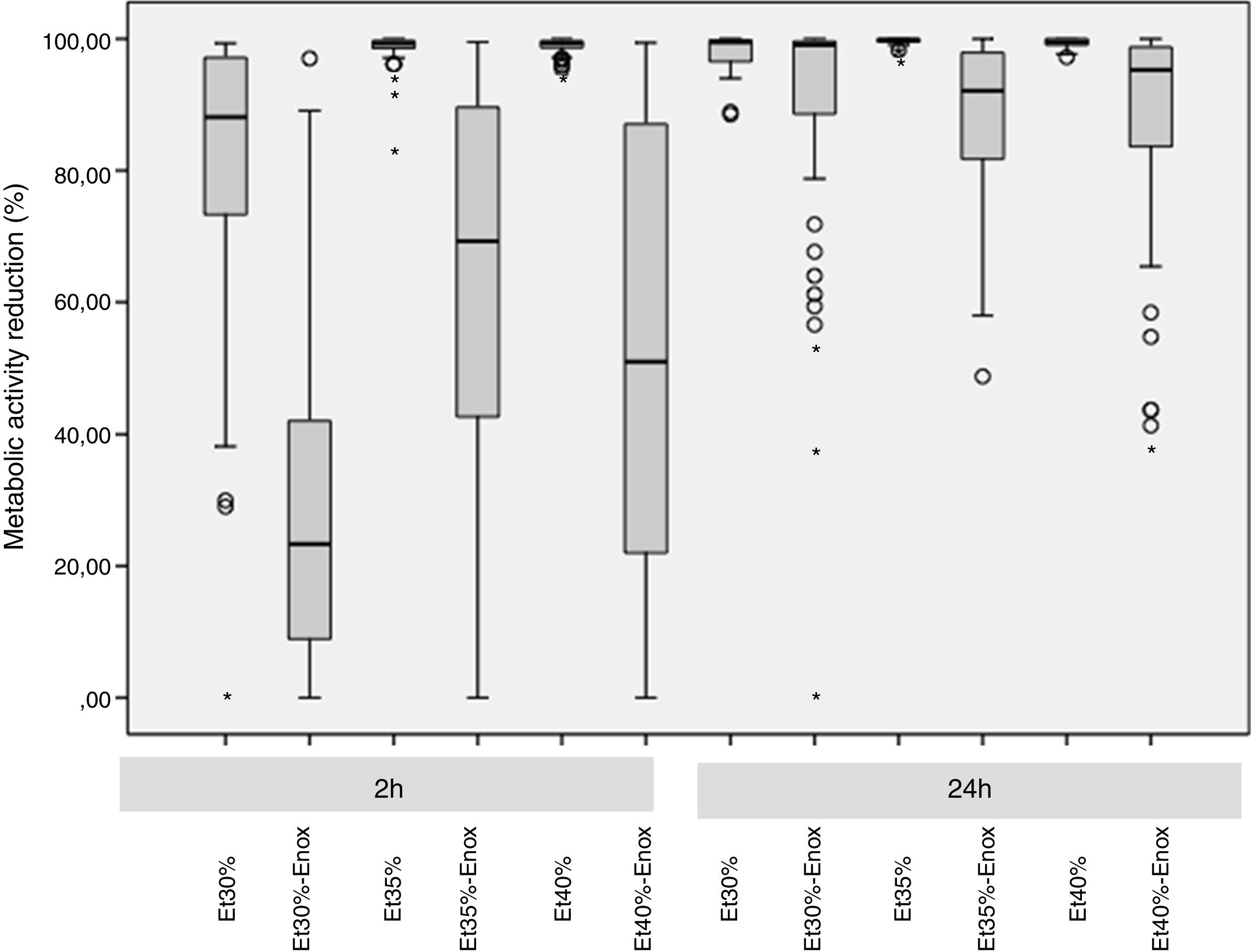
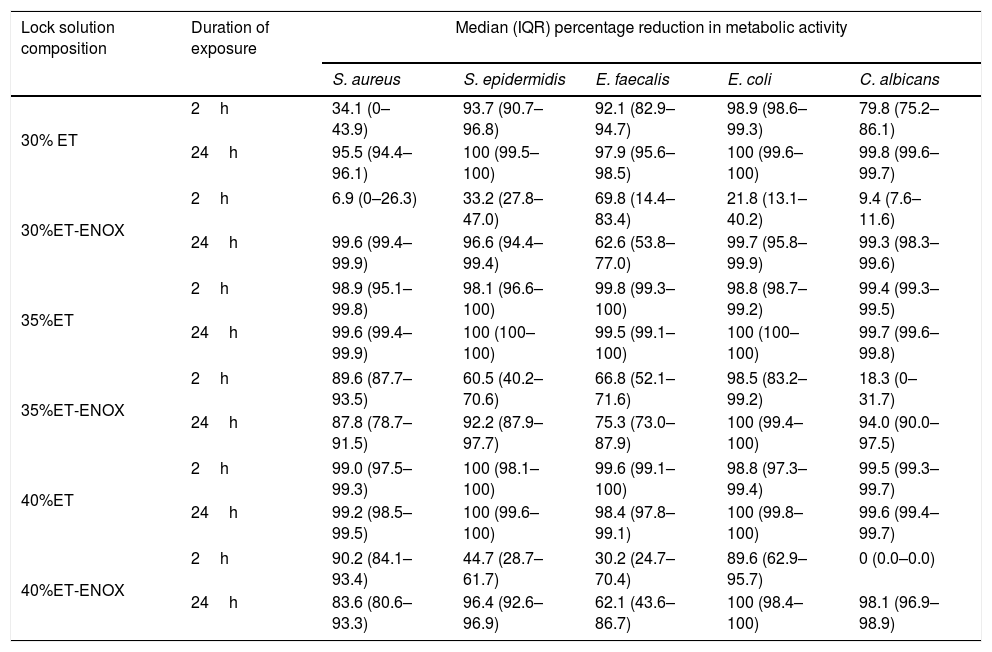
ResultsThe overall median reduction in metabolic activity is shown in Fig. 1. The median reduction obtained for each strain tested with all the solutions at different times of exposure is shown in Table 1.
Reduction in metabolic activity of the biofilm-forming strains after treatment with an ethanol-based lock solution.
| Lock solution composition | Duration of exposure | Median (IQR) percentage reduction in metabolic activity | ||||
|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. faecalis | E. coli | C. albicans | ||
| 30% ET | 2h | 34.1 (0–43.9) | 93.7 (90.7–96.8) | 92.1 (82.9–94.7) | 98.9 (98.6–99.3) | 79.8 (75.2–86.1) |
| 24h | 95.5 (94.4–96.1) | 100 (99.5–100) | 97.9 (95.6–98.5) | 100 (99.6–100) | 99.8 (99.6–99.7) | |
| 30%ET-ENOX | 2h | 6.9 (0–26.3) | 33.2 (27.8–47.0) | 69.8 (14.4–83.4) | 21.8 (13.1–40.2) | 9.4 (7.6–11.6) |
| 24h | 99.6 (99.4–99.9) | 96.6 (94.4–99.4) | 62.6 (53.8–77.0) | 99.7 (95.8–99.9) | 99.3 (98.3–99.6) | |
| 35%ET | 2h | 98.9 (95.1–99.8) | 98.1 (96.6–100) | 99.8 (99.3–100) | 98.8 (98.7–99.2) | 99.4 (99.3–99.5) |
| 24h | 99.6 (99.4–99.9) | 100 (100–100) | 99.5 (99.1–100) | 100 (100–100) | 99.7 (99.6–99.8) | |
| 35%ET-ENOX | 2h | 89.6 (87.7–93.5) | 60.5 (40.2–70.6) | 66.8 (52.1–71.6) | 98.5 (83.2–99.2) | 18.3 (0–31.7) |
| 24h | 87.8 (78.7–91.5) | 92.2 (87.9–97.7) | 75.3 (73.0–87.9) | 100 (99.4–100) | 94.0 (90.0–97.5) | |
| 40%ET | 2h | 99.0 (97.5–99.3) | 100 (98.1–100) | 99.6 (99.1–100) | 98.8 (97.3–99.4) | 99.5 (99.3–99.7) |
| 24h | 99.2 (98.5–99.5) | 100 (99.6–100) | 98.4 (97.8–99.1) | 100 (99.8–100) | 99.6 (99.4–99.7) | |
| 40%ET-ENOX | 2h | 90.2 (84.1–93.4) | 44.7 (28.7–61.7) | 30.2 (24.7–70.4) | 89.6 (62.9–95.7) | 0 (0.0–0.0) |
| 24h | 83.6 (80.6–93.3) | 96.4 (92.6–96.9) | 62.1 (43.6–86.7) | 100 (98.4–100) | 98.1 (96.9–98.9) | |
ET, ethanol; ENOX, enoxaparin; IQR, interquartile range.
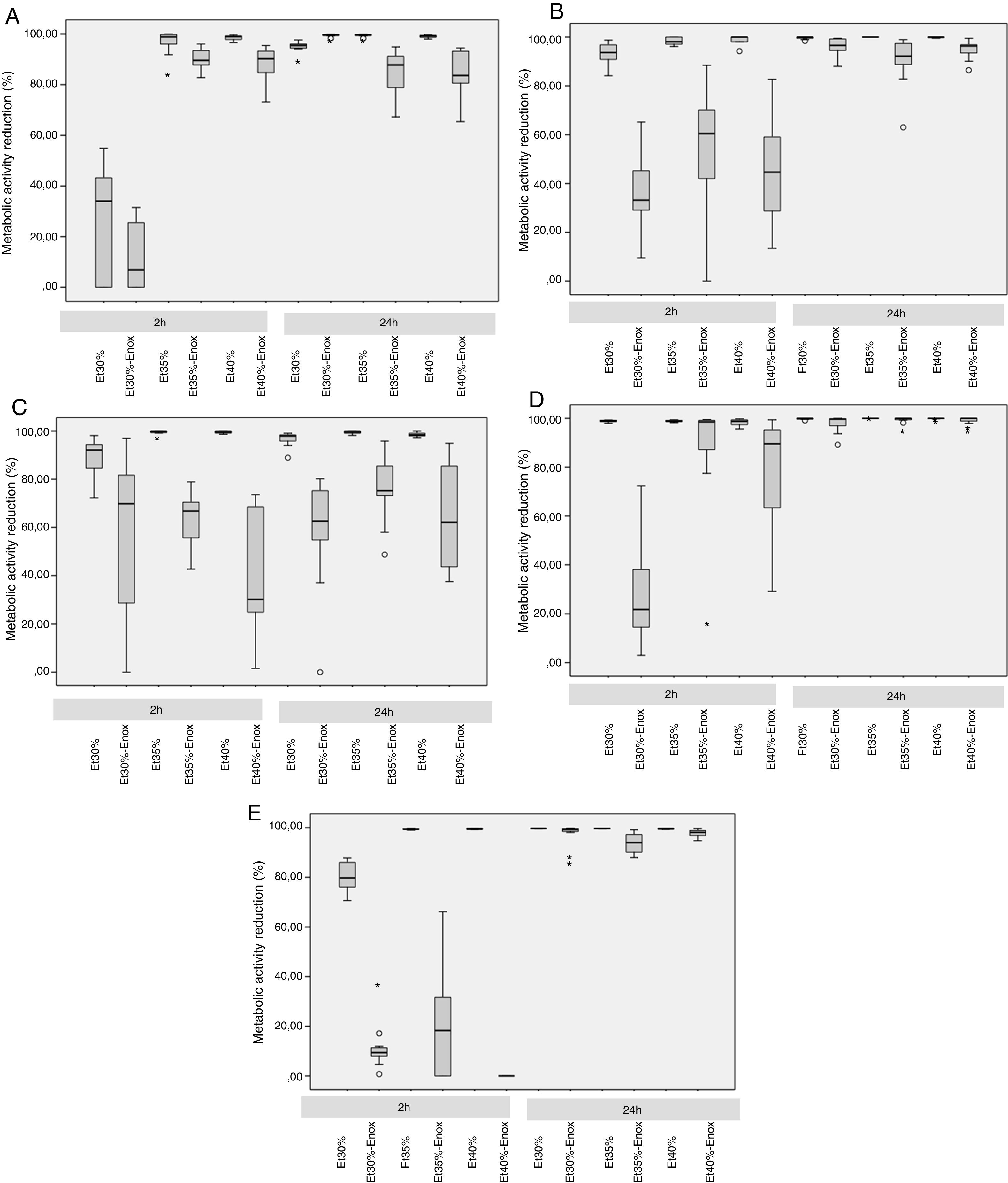
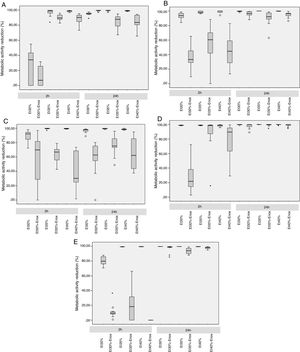
In the 2-h regimen, therapy was successful in all the microorganisms tested when the solution of ≥35% ethanol alone was used. For 30% ethanol, this reduction was only achieved for S. epidermidis and E. coli (Fig. 2B and D). No significant differences in reduction were found between ethanol 35% and 40% (p>0.05).
Median reduction in metabolic activity of the various biofilm-forming strains after treatment with an ethanol-based lock solution. (A) Staphylococcus aureus ATCC25923. (B) Staphylococcus epidermidis (clinical strain). (C) Enterococcus faecalis ATCC33186. (D) Escherichia coli ATCC25922. (E) Candida albicans ATCC14058. Asterics correspond to extreme outliers.
Concentrations of 30% ethanol–enoxaparin reduced the overall median metabolic activity by up to 36%. At 35% ethanol–enoxaparin, the median reduction in metabolic activity ranged from 49% to 89.2%. The same range was observed when 40% ethanol–enoxaparin solutions were used (Fig. 1). However, no reduction was found for C. albicans with any of the 3 concentrations of ethanol–enoxaparin tested (Fig. 2E).
Twenty-four-hour regimenIn the 24-h regimen, therapy was successful in all the microorganisms tested at all the ethanol concentrations (Fig. 1). However, when ethanol was combined with enoxaparin, a significant reduction in metabolic activity was observed only for S. epidermidis, E. coli, and C. albicans at all the ethanol concentrations (p<0.001) (Fig. 2B, D, and E). In contrast, with S. aureus and E. faecalis, the median reduction in metabolic activity did not reach 90% when they were treated with solutions of ethanol at any concentration combined with enoxaparin (Fig. 2A and C).
DiscussionOur data showed that enoxaparin used as an adjuvant anticoagulant in ethanol-based lock solutions negatively affects the anti-biofilm activity of ethanol. Higher concentrations of ethanol were required to obtain better percentage reductions in metabolic activity after combination with enoxaparin.
The consequences of C-RBSI after insertion of a central venous catheter (CVC) in ICU patients can be fatal.22 Guidelines recommend removing the catheter when there is suspicion of C-RBSI. However, in situations where catheter withdrawal is not possible, the combination of systemic therapy and ALT can be useful.8,10,23 Several studies have shown antiseptic lock solutions such as ethanol to be a sufficiently efficacious alternative to ALTs.9,11,16 However, the heterogeneity of the study designs prevents consensus on the appropriate concentration and regimen to be used.24 Most studies showed the best activity with 70% ethanol. However, it has also been demonstrated that 70% ethanol was not only unsafe for patients, but also disrupted the catheter surface.25,26 In our study, we showed that 35% or 40% ethanol alone was sufficiently high for a >90% reduction in the metabolic activity of the biofilm of all the tested microorganisms in a 2-h lock solution regimen (p<0.001).
When ALT is administered for >2h, the solution must contain anticoagulants in order to prevent catheter occlusion. The most popular anticoagulant in lock solutions is heparin, both in its unfractionated form (UFH) and low-molecular-weight form (LMWH).27 Enoxaparin is the most representative LMWH and is replacing UFH owing to its more predictable pharmacokinetic profile and ease of use, although data on its effect and stability in ethanol lock solutions are scarce.28 Calvet et al. demonstrated that enoxaparin was stable in 40% ethanol and that it had only a marginal impact on the catheter surface. Moreover, in a study by Balestrino et al., the efficacy of enoxaparin 400IU/ml and 40% ethanol made it possible to eradicate the biofilm of S. aureus and C. albicans. In addition, the authors confirmed that the integrity of the catheter was not affected.16,17 Biofilms were formed and treated in microfermentors containing segments of silicon catheters, and a significant reduction in the viability of cells (in colony-forming units) was achieved for all their strains when a 24-h regimen was followed, even in S. aureus. In contrast to these results, we found that 60IU of enoxaparin altered the anti-biofilm activity of ethanol in S. aureus and E. faecalis for the 24-h therapy. However, the combination worked properly with S. epidermidis, C. albicans, and E. coli biofilms. Moreover, the shortest regimen used by Balestrino et al. was 4h, compared with 2h in our study, thus showing that the effect of ethanol–enoxaparin solutions was not only ethanol concentration–dependent but also time-dependent. However, although the methodology used was different, both XTT and colony-forming unit counts could help to assess the in vitro anti-biofilm activity of ethanol.
Based on our data, we consider that a 2-h regimen of either 35% or 40% ethanol alone could be used until the microorganism has been identified. However, if a 24-h regimen of ethanol is used, anticoagulants other than enoxaparin should be administered, at least in the case of S. aureus and E. faecalis infections.
Although ours is one of the first studies to assess the anti-biofilm activity of ethanol combined with enoxaparin as a lock solution, it has some limitations. First, our methodology using a static in vitro model was only based on calculating the percentage reduction in metabolic activity as an indirect measure for biofilm reduction. Besides, we also used a clinical strain of S. epidermidis instead of an ATCC which could have different behaviour. Therefore, future studies including more clinical strains must be performed to assess the correlation between metabolic activity and cell viability or re-growth assays.
ConclusionBased on our in vitro results, enoxaparin could negatively affect the anti-biofilm properties of ethanol. We consider that a 35% ethanol-based lock solution is appropriate to be used as a lock therapy. For therapies of >2h, anticoagulants other than enoxaparin may be used at least in the case of S. aureus and E. faecalis infections.
Financial supportM. Guembe is supported by the Miguel Servet Program (ISCIIIMICINN, CP13/00268) from the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. Beatriz Alonso is supported by the Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid and Fondo Social Europeo (PEJ15/BIO/AI-0406). The study was partially funded by the European Regional Development Fund (FEDER) “A way of making Europe”.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Thomas O’Boyle for his help in the preparation of the manuscript.