Periodic outbreaks of hepatitis A (HAV) infection in men who have sex with men (MSM) have been reported. Low vaccination uptake in HIV-infected individuals could drive new outbreaks. We aimed at evaluating the incidence of and risk factors for HAV infection in people living with HIV (PLWH) in our area. We also assessed the rates of HAV vaccination.
MethodsThis was a prospective cohort study. 915 patients were included, 272 (30%) of them were anti-HAV seronegative at baseline.
ResultsTwenty-six (9.6%) susceptible individuals became infected. Incident cases peaked in 2009–2010 and 2017–2018. Incident HAV infection was independently associated with MSM [adjusted odds ratio (95% confidence ratio): 4.39 (1.35–14.27), p=0.014]. One hundred and five (38.6%) HAV seronegative patients were vaccinated, 21 (20%) of them did not respond, and one (1%) patient lost immunity against HAV. Four (29%) non-responders to vaccination showed incident HAV 5–9 years afterwards.
ConclusionsThe incidence of HAV infection in a cohort of well-controlled PLWH remains low and stable, with intermittent outbreaks involving mainly non-immunized MSM. A significant proportion of PLWH remain susceptible to HAV infection due to insufficient vaccine uptake and limited response to vaccination. Importantly, patients not responding to HAV vaccination continue at risk of infection.
Se han notificado brotes periódicos de infección por hepatitisA (VHA) en hombres que practican sexo con hombres (HSH). La baja tasa de vacunación en personas infectadas por el VIH podría dar lugar a nuevos brotes.
ObjetivoEvaluar la incidencia y los factores de riesgo de la infección por VHA en personas que viven con el VIH (PVVS) en nuestra área. Se evaluaron también las tasas de vacunación frente al VHA.
MétodosEstudio de cohortes prospectivo en el que se incluyeron 915 pacientes, 272 (30%) de los cuales eran seronegativos frente al VHA al inicio del estudio.
ResultadosVeintiséis (9,6%) individuos susceptibles se infectaron. Los casos incidentes alcanzaron su punto máximo en 2009-2010 y en 2017-2018. La infección incidente por VHA se asoció de forma independiente con HSH (odds ratio ajustada [índice de confianza del 95%]: 4,39 [1,35-14,27], p=0,014). Ciento cinco (38,6%) pacientes seronegativos frente al VHA fueron vacunados, 21 (20%) de ellos no respondieron y un paciente (1%) perdió la inmunidad frente al VHA. Cuatro (29%) pacientes que no respondieron a la vacunación presentaron infección por el VHA entre 5 y 9años después.
ConclusionesLa incidencia de la infección por VHA en una cohorte de PVVS bien controlada sigue siendo baja y estable, con brotes intermitentes que afectan principalmente a HSH no inmunizados. Una proporción significativa de PVVS sigue siendo susceptible a la infección por VHA debido a la insuficiente vacunación y a la respuesta limitada a la misma. Es importante destacar que los pacientes que no responden a la vacunación frente al VHA siguen estando en riesgo de infección.
WHO estimates that approximately 1.5 million people become infected by hepatitis A virus (HAV) every year.1 HAV is primarily spread by the fecal-oral route through person-to-person contact or by contaminated food or water. Due to an improvement of health conditions and also because of the socioeconomic development, the incidence of HAV infection is low in European countries.2,3 However, spikes in the incidence rate of HAV infection have been detected in the adult population. Epidemic outbreaks of the disease have occurred in Europe4–6 and USA7,8 mainly among men who have sex with men (MSM) infected or not with HIV.6,9
Although vaccination against HAV is an effective way to prevent individual infections and to reduce the impact of outbreaks, its recommendation for adults is not included in most of the vaccination policies in European countries and USA.10,11 Even so, when HAV vaccination is recommended in vaccination programs, it is mostly approved for high-risk groups of people, where HIV-infected patients are not included as one of them until recently only in some countries, in 2018 in the case of Spain.12 For that reason, low rates of vaccination against HAV have been reported among risk groups as HIV-infected patients, MSM or injecting drugs users (IDUs).7,8,13
Incidence studies based on serology and conducted in large prospective HIV-infected cohorts are needed in order to a better estimate of the real incidence of and risk factors for HAV infection among HIV-infected patients. Most importantly, these studies could identify people vaccinated that do not develop immunological response against the virus, leaving this group of people at risk of infection. To the best of our knowledge, the information about factors associated with HAV infection come from case reports and cross-sectional studies7,8,13 with the subsequent underestimation of the real incidence of the infection.
Because of this, we aimed at evaluating the incidence of and risk factors for HAV infection in HIV-infected patients in our area in recent years. We also assessed the rates of HAV vaccination in this population.
MethodsStudy design and patientsThis was a prospective cohort study. The cohort includes all HIV-infected patients attending the Unit of Infectious Diseases in the Hospital Universitario Virgen de Valme from January 2008 to August 2019. Patients were seen in scheduled clinical visits at least every 6 months. At each visit, epidemiological, clinical and laboratory data were recorded. A serum sample was collected and stored in each programmed visit. All included patients had two or more frozen serum samples available collected at least 12 months apart. Individuals with only one serum sample were excluded from the study.
Definitions and proceduresPatients were classified as incident HAV infection cases if they had a baseline negative serum HAV antibody test and detectable serum HAV antibodies at the end of follow-up with an antibody cut-off >20UI/mL to be considered positive titer. For incident HAV cases, the year of infection was determined by testing sera stored every year since baseline, in order to detect the year of seroconversion. Patients vaccinated against HAV during the follow-up were censored at the date of seroconversion post-vaccination.
Sera remained frozen at −80°C until thawing for the determination of HAV antibodies. HAV antibodies were measured using a commercial bioluminescence immunoassay (LIAISON Anti-HAV (310170), DiaSorin S.p.A., Saluggia, Vercelli, Italy).
Vaccination took place at the Unit of Preventive Medicine in our Hospital. Following the national recommendations, patients received 2 doses of the vaccine 6 months apart. Patients with only one dose were considered incomplete vaccinations.
Statistical analysisFor descriptive analysis, continuous variables were expressed as median (Q1–Q3) and categorical variables as frequencies (percentage). The χ2 test or Fisher's exact test were used to compare the distribution of categorical variables. The Student's t-test or the Mann–Whitney U test were applied to compare continuous variables. Global incidence and biannual incidence were calculated along with 95% confidence interval (95% CI). Binary logistic regression models were elaborated to assess the factors independently associated with HAV seroconversion among susceptible patients who had not been vaccinated. In those analyses, variables related to this condition with a univariate p value <0.2, as well as age and sex, were included. Differences were considered significant for p values ≤0.05. The incidence of infection during outbreak periods (2009–2010 and 2017–2018) were compared with that observed in the period of lower incidence of infection detected (2013–2014) in order to find out possible different features between periods. All analyses were carried out using the SPSS software 25.0 (IBM Corporation, Somers, New York City, New York, USA).
Ethical issuesThis study was designed and performed according to the Helsinki declaration and was approved by the ethics committee of the Hospital Universitario Virgen de Valme (Seville, Spain). Informed consent was obtained from all individuals.
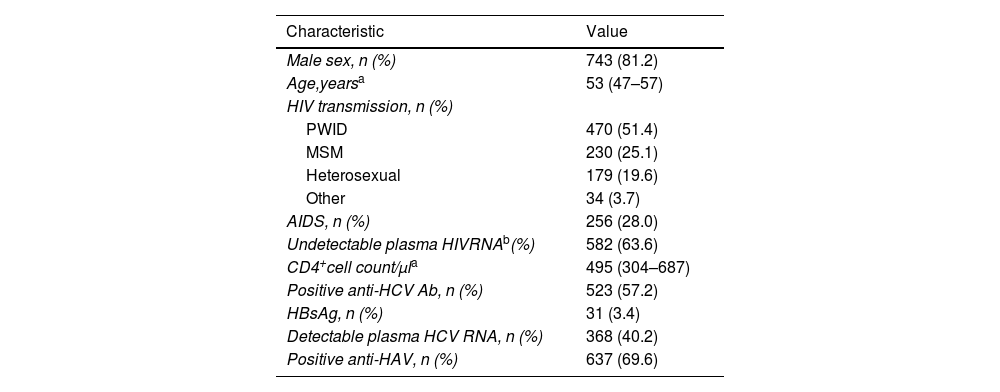
ResultsCharacteristics of the populationOf the 933 patients included in the cohort, 915 finally met the inclusion criteria. The baseline characteristics of the patients are summarized in Table 1. Most individuals showed a good immunological status (Table 1). The median time of follow-up was 7.7 (3.9–9.6) years. There were 147 (16.1%) patients lost to follow-up and 73 (8%) individuals died during the study.
Baseline characteristics of the study population (n=915).
| Characteristic | Value |
|---|---|
| Male sex, n (%) | 743 (81.2) |
| Age,yearsa | 53 (47–57) |
| HIV transmission, n (%) | |
| PWID | 470 (51.4) |
| MSM | 230 (25.1) |
| Heterosexual | 179 (19.6) |
| Other | 34 (3.7) |
| AIDS, n (%) | 256 (28.0) |
| Undetectable plasma HIVRNAb(%) | 582 (63.6) |
| CD4+cell count/μla | 495 (304–687) |
| Positive anti-HCV Ab, n (%) | 523 (57.2) |
| HBsAg, n (%) | 31 (3.4) |
| Detectable plasma HCV RNA, n (%) | 368 (40.2) |
| Positive anti-HAV, n (%) | 637 (69.6) |
Abbreviations: PWID: people who inject drugs; MSM: men who have sex with men; AIDS: acquired immunodeficiency syndrome; HCV: hepatitis C virus; Ab: antibody; HBsAg: surface antigen of the hepatitis B virus.
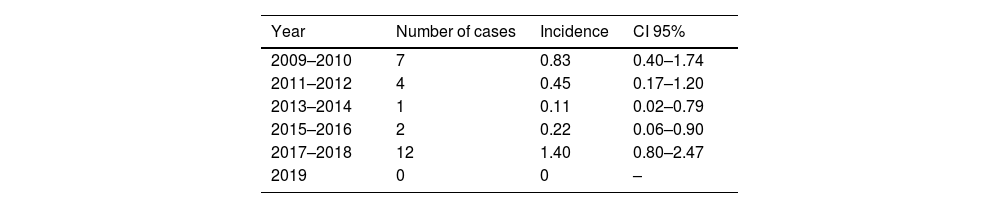
At baseline, 272 (30%) subjects had a negative HAV serology and 26 (9.5%) of them became infected with HAV during the study period. Of those, 18 (69.2%) were asymptomatic and 8 (30.8%) developed symptoms. Only one (12.5%) of the symptomatic patients developed a severe acute hepatitis and needed admission. All susceptible patients had a serum sample yearly until the date of seroconversion associated with infection or vaccination. The median time of follow-up among susceptible individuals was 6.2 (3.0–9.6) years. Among patients with HAV incident infection, 15 (58%) were MSM and 11 (42%) non-MSM individuals (p=0.267). Biannual incident cases among all susceptible individuals are summarized in Fig. 1. Annual incidence was 1.54 (95% CI, 1.05–2.26) per 100 persons-year. Biannual incidence is shown in Table 2. Incident cases peaked in 2009–2010 and 2017–2018 (Fig. 1). MSM was de risk group more represented in the outbreaks compared with the valley period of 2013–2014 [(2009–2010: 3 (6.8%) vs. 2013–2014: 1 (1.3%), p=0.102) (2017–2018: 7 (8.0%) vs. 2013–2014: 1 (1.3%), p=0.045)].
The factors associated with incident infection among unvaccinated patients are shown in Table 3. After multivariate analysis, adjusted by age, sex and CDC stage, MSM group and age were independently associated with incident HAV infection (Table 3).
Univariate and multivariate analysis of risk factors associated with incident HAV infection among unvaccinated individuals (n=167).
| Parameter | N | Frequency of HAV infectionN (%) | p univariate | AOR (95% CI) | p multivariate |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 43 | 2 (4.7%) | 0.022 | 2.99 (0.61–14.73) | 0.178 |
| Male | 124 | 24 (19.4%) | |||
| Agea | |||||
| <46 | 80 | 14 (17.5%) | 0.509 | 1.08 (1.02–1.14) | |
| ≥46 | 87 | 12 (13.8%) | 0.008 | ||
| Risk group | |||||
| MSM | 63 | 15 (23.8%) | 0.022 | 4.39 (1.35–14.27) | 0.014 |
| No MSM | 104 | 11 (10.6%) | |||
| Plasma HIVRNAb | |||||
| Detectable | 114 | 17 (14.9%) | 0.731 | – | – |
| Undetectable | 53 | 9 (17%) | |||
| CDC stage | |||||
| C | 36 | 3 (8.3%) | 0.170 | 0.29 (0.07–1.28) | 0.109 |
| A or B | 131 | 23 (17.6%) | |||
| CD4 cell count/ml | |||||
| <350 | 49 | 9 (18.4%) | 0.603 | – | – |
| >350 | 118 | 17 (14.4%) | |||
Abbreviations: MSM: men who have sex with men; CDC stage: centers of disease control and prevention stage; AOR: adjusted odd ratio.
Among 272 patients with negative baseline HAV serology, 105 (39%) were vaccinated against HAV along the study period. Out of those, 65 (61.9%) were MSM. Vaccinated patients received the first dose of vaccine a median (Q1–Q3) of 19 (7–75) months after the baseline date. Seventy-six (72.4%) individuals completed the vaccination schedule with 2 doses of the vaccine. Sixty-six (87%) of them showed seroconversion after HAV vaccination. Most patients with adequate vaccination showed persistent serum antibodies, but one (1.5%) individual showed seroreversion 4 years after successful vaccination. Among 10 (13%) non-responders to adequate vaccination, there were three (30%) patients who seroconverted five to nine years after vaccination (Table 4) and they were considered as infections. Among 28 (27%) patients with an incomplete HAV vaccination schedule, 11 (39%) did not show anti-HAV seroconversion after the first and only vaccine dose. One (9.1%) of them showed anti-HAV serocoversion five years after vaccination with only one dose and he was also considered as an infection. The overall incidence of HAV infection for vaccinated patients was 3.8% (4 out of 105) with all of them developing asymptomatic infections.
Characteristics of vaccinated individuals who became infected with HAV (n=4).
| First dose date | Second dose date | Time between the last dose and the date of seroconversiona | Seroconversion year | |
|---|---|---|---|---|
| Patient 1 | June 22nd, 2011 | December 21st, 2011 | 6 years | April, 2017 |
| Patient 2 | May 27th, 2008 | October 21st, 2008 | 9 years | September, 2017 |
| Patient 3 | January 20th, 2010 | November 22nd, 2010 | 8 years | June, 2018 |
| Patient 4 | March 22nd, 2012 | – | 5 years | March, 2017 |
Patient 4 was incompletely vaccinated, received only the first dose.
We found that the incidence of HAV infection, confirmed by serological testing, among a well-controlled HIV-infected cohort remains low and steady, with periodical outbreaks involving mainly MSM. The fact that a significant proportion of these patients remain susceptible to HAV infection leads to these outbreaks. Insufficient vaccine coverage and limited response to vaccination underlies the elevated susceptibility to HAV infection in this population. In addition, these results prove that patients not responding to anti-HAV vaccination continue at risk of infection.
Our outbreaks observations are consistent with previous data in different areas in Europe mainly observed in unvaccinated MSM, regardless whether they were infected 9 or not by HIV.5,14 Most incident HAV infections in our study were detected in MSM, as in previous reports.4–9,13,15 During our period of study two outbreaks were reported in different countries in 2008–200916 and in 2016–2017.5,7,9,16 Both international outbreaks overlap in time with the outbreaks observed in our study pointing out that our serological study is in line with those surveys in the rest of Europe and USA.
In agreement with previous observations, we found a significant rate of HAV vaccine failure. Immunosuppression has been reported to influence HAV vaccine response in HIV infection. CD4 cell counts lower than 200cells/mcL and low CD4/CD8 ratio have been associated with poorer response.17,18 For HIV viremia, there are contradictory results, with studies showing an association of higher levels of viremia with lack of seroconversion after vaccination,19,20 and other studies reporting no relationship with response to vaccination.17,21 In any case, lower protection rates following vaccination are observed in HIV infection,22,23 where also specific antibodies are lower among HIV positive individuals compared with the general population.24 Although it is not clear whether those individuals should undergo HAV revaccination, the loss of immunity may be addressed using additional boosters.25–29 Moreover, high seroconversion rates in HIV-infected population with one-dose of HAV inactivated vaccine after losing anti-HAV following primary vaccination have been reported.27 Thus, follow-up monitoring of serological response after vaccination to evaluate the need of additional booster vaccination among HIV-infected population should be implemented.
After adequate HAV vaccination, the majority of patients with initial response to vaccination showed long-term persistence of antibodies. Only one patient lost anti-HAV antibodies four years after vaccination. This potential loss of response over time indicates that periodic reassessment HAV serology is needed for HIV-infected patients. However, the more frequent reason for lack of seroprotection among vaccinated patients was an incomplete schedule of vaccination. Nearly one third of the individuals who started the vaccination program did not receive the second dose of vaccine. Potential explanations for this failure to complete the vaccination program include the loss of adherence to the program follow-up. The tertiary care centered vaccination, as opposed to administration in primary care settings, is another likely barrier together with the fact that HAV vaccination was not recommended until 2018 in our country for HIV positive patients.
Asymptomatic infections are the common presentation in children. On the contrary, approximately 30% of patients older than 6 years develop symptoms.24 Regarding HIV-infected patients, the information is scarce about the possible differences with HIV-uninfected individuals. A small study suggested no differences in the frequency or severity of symptoms.30 In the present study, we found that one third of the patients with HAV seroconversion developed symptoms, in line with the clinical picture in the general population. Interestingly, patients with complete or incomplete vaccination who were infected by HAV did not develop symptoms. This may indicate that some low level of immunity among vaccinated HIV-infected patients, below the threshold of antibody titer considered to indicate seroprotection, could induce a milder hepatitis A course among.
Our study presents several limitations. First, this was a cohort study based on the analysis of stored sera during a long period of observation. Should sera had been inconsistently gathered, the estimations of incidence would have been inaccurate. However, we could evaluate yearly serum samples of 94% patients in the cohort. This is a strength of the study. Second, detailed sexual habits were not assessed, which precludes an evaluation of sexual risk factors associated with HAV infection. Third, 6.7% vaccinated individuals lacked serological follow-up. The prolonged period of follow up and the annual serology performed within a cohort of HIV-infected patients are strengths of this study.
In conclusion, insufficient immunization against HAV in HIV-infected people leads to periodic hepatitis A outbreaks. Importantly, patients without response to HAV vaccination are at risk of incident infection. Strategies to implement widespread HAV vaccination are needed for HIV-infected people and particularly MSM HIV-infected patients.
Data availabilityThe datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributionsJuan Macias (J.M.) and Marta Fernandez-Fuertes (M.F.F.) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Juan A Pineda (J.A.P.) and Luis M. Real (L.M.R.).
Acquisition, analysis, or interpretation of data: J.M., M.F.F., Anaïs Corma-Gomez (A.C.G.), Adolfo de Salazar (A.S.).
Statistical analysis: J.A.P. and L.M.R.
Drafting of the manuscript: J.M. and M.F.F.
Critical revision of the manuscript for important intellectual content: J.M., M.F.F., A.C.G., A.S., Alejandro González-Serna (A.G.S.), Pilar RINCON (P.R.), Maria J. Arriaza-Estevez (M.J.A.E.), Ana Fuentes-Lopez (A.F.L.), L.M.R., and J.A.P.
Obtained funding: J.M., A.C.G, A.G.S. and J.A.P.
Study supervision: J.A.P. and L.M.R.
FundingThis work was supported in part by the Instituto de Salud Carlos III (Project’PI16/01443’), integrated in the national I+D+i 2013-2016 and co-funded by the European Union (ERDF/ESF,“Investing in your future”), by the Spanish Network for AIDS investigation (RIS)(http://www.red.es/redes/inicio) (RD16/0025/0010), as a part of the Nacional I+D+I, ISCIII Subdirección General de Evaluación and the European Fund for Development of Regions (FEDER). This research was also supported by CIBER -Consorcio Centro de Investigación Biomédica en Red-(CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea-NextGenerationEU. JAP has received a research extension grant from the Programa de Intensificación de la Actividad de Investigación del Servicio Nacional de Salud Carlos III (I3SNS). JM has received a research extension grant from Consejería de Salud, Junta de Andalucía (A1-0060-2021). A.C-G has received a research extension grant Acción B, Acción para el Refuerzo de la Actividad Investigadora en las Unidades Clínicas del Servicio Andaluz de Salud 2021, Clínicos Investigadores (grant number B-0061-2021). This work was supported by the Ministry of Science, Innovation and Universities of Spain for A.G.-S who is recipient of a Miguel Servet Research Contract (CP18/00146), co-financed by the FSE’El Fondo Social Europeo invierte en tu futuro’.
Conflict of interestsJM has been an investigator in clinical trials supported by Bristol-Myers Squibb, Gilead and Merck Sharp & Dome. He has received lectures fees from Gilead, Bristol-Myers Squibb, and Merck Sharp & Dome, and consulting fees from Bristol Myers-Squibb, Gilead, and Merck Sharp & Dome. JAP reports having received consulting fees from Bristol-Myers Squibb, Abbvie, ViiV Healthcare, Gilead, Merck Sharp & Dome, and Janssen Cilag. He has received research support from Bristol-Myers Squibb, ViiV Healthcare, Abbvie, Merck Sharp & Dome, Janssen Cilag and Gilead and has received lecture fees from Abbvie, Bristol-Myers Squibb, ViiV Healthcare, Merck Sharp & Dome, Abbvie, Janssen Cilag, and Gilead. ACG has received lecture fees from Gilead, Merck Sharp & Dome and Abbvie. The remaining authors report no conflict of interest.