Microbiological Procedure number 61 of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) [Spanish Society of Infectious Diseases and Clinical Microbiology] entitled “Microbiological methods for monitoring the cleaning, disinfection and sterilisation of medical devices” focuses on reusable medical devices qualified as semi-critical or medium risk; those that come into contact with the mucous membranes without penetrating sterile tissues and which require at least high-level disinfection. With this in mind, we would like to present a study about microbial contamination of liposomal amphotericin B nebulising equipment in lung transplant patients.
Nebulisers are devices for administering a drug through the airways by aerosol delivery. In the context of lung transplantation, home-administered prophylaxis with nebulised amphotericin B is effectively used for the prevention of infection by Aspergillus spp.1 These devices are a potential source of infection in the event of microbial contamination.2 Very few studies have assessed the incidence of microbial contamination in nebulisers and the clinical repercussions,3 particularly in lung transplantation.4 Moreover, there is a lack of consensus on the standardisation of cleaning and disinfection procedures and microbiological monitoring of these devices.5 The aim of this study is to estimate the prevalence of microbial contamination in amphotericin B nebulising systems used by lung transplant patients, and to assess the impact on the patient's microbiology.
When the prophylaxis was starting, all patients were instructed on how to adequately clean and disinfect the nebuliser (washing with a brush, soap and water and immersion in 1% sodium hypochlorite solution). After the transplant, the patients had visits every month for the first year, every two months in the second year and then every three to four months. Sputum cultures were taken at these visits if the patient expectorated and fibreoptic bronchoscopy was also occasionally performed.
In 2012–2014, the nebulisation equipment of a cohort of lung transplant patients was sampled. We studied 75 cultures from 71 patients; 42 males, with a mean age of 49 (range: 5–64 years), and 29 females, with a mean age of 51 (22–66 years). Samples were taken from the pipette, the reservoir and tubing of the device. The median time between transplantation and collection of the sample was 666 days (range: 68–1470 days). The results of the cultures of respiratory samples collected in the three months before and after the sampling of the nebuliser were also reviewed.
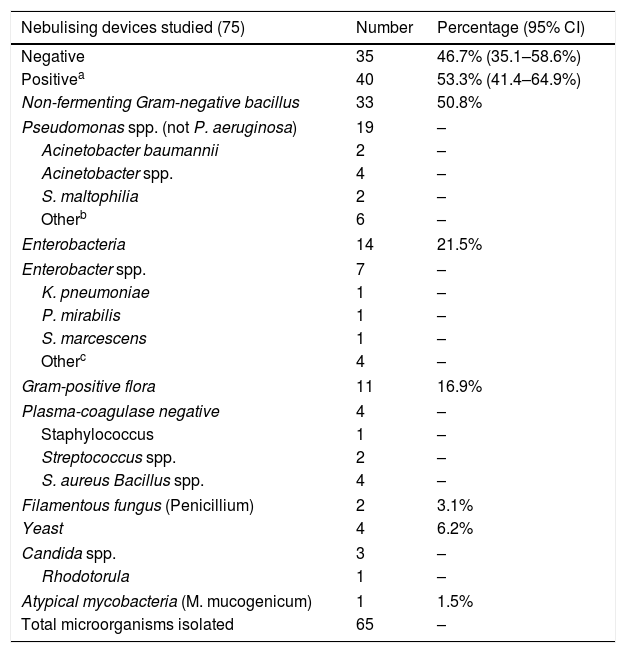
Microbial contamination was detected in 53.3% of the nebulisers (Table 1). The contamination rate was significantly higher in the pipette and the reservoir (50.7%) than in the device tubing (11.5%) (p<0.005). Pseudomonas aeruginosa was not isolated in any of the nebulisers.
Microorganisms isolated in the nebulisers.
| Nebulising devices studied (75) | Number | Percentage (95% CI) |
|---|---|---|
| Negative | 35 | 46.7% (35.1–58.6%) |
| Positivea | 40 | 53.3% (41.4–64.9%) |
| Non-fermenting Gram-negative bacillus | 33 | 50.8% |
| Pseudomonas spp. (not P. aeruginosa) | 19 | – |
| Acinetobacter baumannii | 2 | – |
| Acinetobacter spp. | 4 | – |
| S. maltophilia | 2 | – |
| Otherb | 6 | – |
| Enterobacteria | 14 | 21.5% |
| Enterobacter spp. | 7 | – |
| K. pneumoniae | 1 | – |
| P. mirabilis | 1 | – |
| S. marcescens | 1 | – |
| Otherc | 4 | – |
| Gram-positive flora | 11 | 16.9% |
| Plasma-coagulase negative | 4 | – |
| Staphylococcus | 1 | – |
| Streptococcus spp. | 2 | – |
| S. aureus Bacillus spp. | 4 | – |
| Filamentous fungus (Penicillium) | 2 | 3.1% |
| Yeast | 4 | 6.2% |
| Candida spp. | 3 | – |
| Rhodotorula | 1 | – |
| Atypical mycobacteria (M. mucogenicum) | 1 | 1.5% |
| Total microorganisms isolated | 65 | – |
Despite the microbial findings, the microorganism isolated in the nebuliser only corresponded with the isolate in respiratory samples in one of the 71 patients studied (1.4%). In this case, Proteus mirabilis was isolated in two sputum samples 14 and 24 days respectively after being found in the nebuliser.
There have been few studies similar to the one we present here, where we estimate the prevalence of microbial contamination in amphotericin B nebulising systems used by lung transplant patients. In a 2005 study4 patient adherence to the cleaning and disinfection protocol was poor, at only 39%. After those findings, patient education on care of the nebulising equipment was intensified.
The aforementioned Microbiological Procedure highlights the magnitude of the problem of nosocomial infection associated with semi-critical devices and the insufficient surveillance to which they are subjected. The procedure also strongly recommends periodic microbiological cultures as part of the quality control of the cleaning and disinfection process. However, this document does not review home-administered nebulised therapy devices in particular clinical contexts, such as cystic fibrosis or lung transplantation, which also require the same considerations.
Despite the limitations of our study, we believe that this work demonstrates the need to standardise appropriate mechanisms for the disinfection of nebuliser devices. We also consider it necessary to protocolise stringent microbiological monitoring of these devices to assess the process; especially in lung transplant and other immunologically compromised patients.
Please cite this article as: Fernández-Huerta M, Escobar R, Monforte V, Rodriguez V. Contaminación microbiana de dispositivos de nebulización de anfotericina B liposomal en pacientes trasplantados pulmonares. Enferm Infecc Microbiol Clin. 2019;37:211–212.