Bacterial sexually transmitted infections (STIs) have an important impact on reproductive health, highlighting the increase in Chlamydia trachomatis infection rates among young people. To reduce the costs of STI detection, the pooling strategy is beneficial for high-throughput tests in low-prevalence populations using non-invasive samples.
Objectives(1) To describe the performance of a 7-STI PCR assay using the pooling of three urine samples to detect C. trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium; (2) to estimate the cost saving of the pooling strategy; (3) to describe the prevalence, risk factors and coinfections of C. trachomatis, N. gonorrhoeae and M. genitalium in young people ≤25 years in Catalonia.
Methodscross-sectional prevalence study conducted in 2016 among young people ≤25 years of age seen in sexual and reproductive health centres throughout Catalonia from pools of three urine samples. A standardized questionnaire was used to collect clinical-epidemiological and behavioural variables.
Results1032 young people were tested. The prevalence of C. trachomatis, N. gonorrhoeae and M. genitalium was 8.5%, 0.6% and 3.5%, respectively. The pooling strategy provided a 33% savings in reagent costs.
ConclusionsThe pooling strategy implemented for epidemiological studies in our context provides a savings that has an impact on the viability of STI detection programmes. In the same way, this study shows that C. trachomatis prevalence continues to increase in this population and, for the first time in Catalonia, the prevalence of M. genitalium in young people is shown.
Las infecciones bacterianas de transmisión sexual (ITS) tienen un impacto importante en la salud reproductiva, destacando el aumento en las tasas de infección por Chlamydia trachomatis entre los jóvenes. Para reducir los costes de detección de las ITS, la estrategia de agrupación de muestras (pooling) es beneficiosa para pruebas de alto rendimiento en poblaciones de baja prevalencia utilizando muestras no invasivas.
Objetivos1) Describir el rendimiento de un ensayo de PCR 7-STI utilizando el pooling de 3 muestras de orina para detectar Chlamydia trachomatis, Neisseria gonorrhoeae y Mycoplasma genitalium; 2) Estimar el ahorro de la estrategia de pooling; 3) Describir la prevalencia, los factores de riesgo y las coinfecciones de Chlamydia trachomatis, Neisseria gonorrhoeae y Mycoplasma genitalium en jóvenes ≤25 años en Cataluña.
MétodosEstudio transversal de prevalencia realizado durante 2016 entre jóvenes ≤25 años atendidos en centros de salud sexual y reproductiva en todo el territorio catalán a partir de pools de 3 muestras de orina. Se utilizó un cuestionario estandarizado para recopilar variables clínico-epidemiológicas y de comportamiento.
ResultadosSe testaron 1032jóvenes. La prevalencia de Chlamydia trachomatis, Neisseria gonorrhoeae y Mycoplasma genitalium fue del 8,5, 0,6 y 3,5%, respectivamente. La estrategia de pooling proporcionó un ahorro del 33% en los costos de reactivo.
ConclusionesLa estrategia de pooling llevado a cabo para estudios epidemiológicos en nuestro contexto proporciona un ahorro que tiene un impacto en la viabilidad de los programas de detección de las ITS. De la misma manera, en este estudio se observa que la prevalencia de Chlamydia trachomatis continúa aumentando en esta población y, por primera vez en Cataluña, se determina la prevalencia de Mycoplasma genitalium en la población joven.
Bacterial sexually transmitted infections (STI) have an important impact on reproductive health worldwide. In Europe, STI risk behaviour and incidence rates are on the rise among young population. Neisseria gonorrhoeae outbreak in very young adolescents has been reported in Catalonia and other STI on the rise.1
Chlamydia trachomatis is the most common STI worldwide, with estimated 105.7 million cases in adults aged 15 and 49.2 In Europe is the most frequently reported with a notification rate of 182 per 100,000 habitants in 2013, 67% of which were reported in young adults <25 years. Gonorrhoea was more frequent in men than in women, predominantly in men who have sex with men (MSM) and 39% being reported among young adults.3 As for the Catalan region, C. trachomatis prevalence has been on the rise among young adults <25 years of age showing a prevalence of 5.8% in 2007 and 8.5% in 2012.4
Most C. trachomatis infection is asymptomatic leading to undiagnosed infection and increased risk of complications. Consequently, although screening programs for C. trachomatis detection are important in order to reduce the C. cases, these can be very expensive.5,6 To reduce costs, previous studies have shown that specimen pooling is beneficial for high-throughput testing in low-prevalence populations (≤10%) using non-invasive specimens such as first void urine by nucleic acid amplification tests (NAATs).7
There are currently no commercially available NAATs approved by the Food and Drug Administration for use with pooled specimens. However, several studies have shown an excellent correlation between the results of pooled specimens and individually tested specimens for C. trachomatis detection.8–12
The aims of this study were (i) to describe the performance characteristics of a PCR 7-STI detection assay using pooled urine specimens for C. trachomatis, N. gonorrhoeae and M. genitalium infections; (ii) to estimate the cost saving of a pooling strategy and (iii) to describe the prevalence, risk factors and co-infections of C. trachomatis, N. gonorrhoeae and M. genitalium among young under 25 years in Catalonia.
MethodsSince 2007, in Catalonia, C. trachomatis prevalence monitoring with bio-behavioural data was carry out in the sentinel population of young people as a part of the integrated epidemiological surveillance system. The objectives of these periodic studies are to know and monitor C. trachomatis prevalence and risk factors of infection among those under 25 years of age attended in sexual and reproductive health centres (ASSIR) and youth care centres.
The study design selected was a cross-sectional observational study of C. trachomatis, N. gonorrhoeae and M. genitalium among young attending 24 ASSIR and 3 youth care centres in Catalonia. These centres are characterized by offering a service of family planning, specific attention to young people, maternal-child care, prevention, diagnosis of gynaecological diseases. Women served mostly in these centres.
The inclusion criteria were sexually active ≤12 months and aged between 16 and 25 years attending at ASSIR and youth care centres for any reason such as general gynaecological attention, STI/HIV screening, symptoms, contraception methods, etc. The recruitment period will be during the first semester of 2016. Epidemiological data was collected using a standardized questionnaire, including socio-demographic and behavioural variables as described by López-Corbeto et al.4 Data anonymity was assured, as no personal identifiers were included in the database. This study was approved by the local Research Ethics Committee: CEICPI-17-217.
Sample size and pooling numberThe optimal number of specimens included in each pool was calculated using 1−(1−P)n formula as explained by Peeling et al.9 were P is the probability a single sample is positive and n is the number of specimens included in each pool. For this study purpose, P was defined as 7% C. trachomatis prevalence as calculated by López-Corbeto et al.4 A number between 2 and 5 specimens per pool were calculated as optimal, choosing 3 as acceptable for the given prevalence and economical resources in our setting.
Specimen analysisFirst void urine specimens were collected from 1032 individuals using the Multicollect Specimen Collection kit (Abbott Molecular Inc, Abbott Park, IL, USA) as recommended by the manufacturer. The pooling of three samples was randomized from the individual samples previously frozen as they arrived at the laboratory in order to establish the runs of consecutive analyses. The pooling was carried out by combining 400microliters of each specimen to yield a total volume of 1200 microliters. 400 microliters of each pool were transferred to a reaction tube containing 2microliters of lysis buffer and 10ml of internal control. The sample was homogenized by vortex agitation and then tubes were incubated for 12min at room temperature. We used NucliSens EasyMAG (BioMérieux) to perform DNA extraction of C. trachomatis and other STI. This system automates an enhanced magnetic silica version of BOOM® technology.13 After nucleic acid extraction, the Anyplex™ II STI-7 detection assay (Seegene, Seoul, Korea) was performed. This multiplex assay used TOCE™ (tagging oligonucleotide cleavage and extension) and DPO™ (dual priming oligonucleotide) technologies and simultaneously detects seven pathogens: C. trachomatis, N. gonorrhoeae, M. genitalium, M. hominis, Ureaplasma urealyticum, Ureaplasma parvum and Trichomonas vaginalis. The semi-quantitative results are obtained based on cyclic-CMTA (catcher melting temperature analysis).14,15 The reaction was performed in a CFX96 real time thermocycler (Bio-Rad, Hercules, CA, USA) following the manufacturer instructions. Each pooled set of specimens was tested, and positive pooled samples were individually tested to identify the positive specimen(s). Pools testing negative or having a positive result to a microorganism other than C. trachomatis, N. gonorrhoeae or M. genitalium were not further tested.
Cost analysisCost analysis was carried out based on reagents direct costs per specimen test. At the moment of study enrolment the cost per test was 24.00€. Percentage of saving was calculated as the difference of the cost required for pooling strategy versus the cost needed for individual testing strategy. No other consumable and personnel cost were considered.
Statistical analysisData analysis was performed using the SPSS statistical package v20.0. Confidence intervals were calculated at a 95% limit to proportions obtained from descriptive variables. C. trachomatis, N. gonorrhoeae and M. genitalium prevalence was calculated as the number of positive specimens for all specimens analyzed. The prevalence of Trichomonas vaginalis was calculated in positive pools of C. trachomatis, N. gonorrhoeae or M. genitalium. A bivariate and multivariate logistic regression analysis was performed to determine C. trachomatis, N. gonorrhoeae and M. genitalium determinants.
ResultsPooling performanceA total of 1032 samples were collected and sent to analyze in the Microbiology Department. Out of all collected samples, 344 pools (3 specimens each) were tested for C. trachomatis, N. gonorrhoeae and M. genitalium. Study STIs were detected in 116 pools from which, 348 additional individual tests were carried out. The overall pooling performance yielded 692 tests (344 initial pooling+348 additional individual tests). The 116 positive pools included: 76 C. trachomatis, 6 N. gonorrhoeae and 34 M. genitalium. Out of the 76 C. trachomatis positive pools, 65 pools were detected with a single positive specimen, 10 pools contained two positive specimens and one pool was positive in all three specimens. As for M. genitalium positive pools, 32 were detected with a single specimen and 2 with two positive specimens. Finally, 6 pools were positive for N. gonorrhoeae carrying a single positive specimen each.
Cost analysis for STI poolingThe study obtained a total of 1032 specimens from which 692 (344 pooled tests+348 individual tests) samples were tested for C. trachomatis, N. gonorrhoeae or M. genitalium. The estimated individual tests cost was 24,768€ versus 16,608€ of pooled specimens accounting to a reagent savings of 33%.
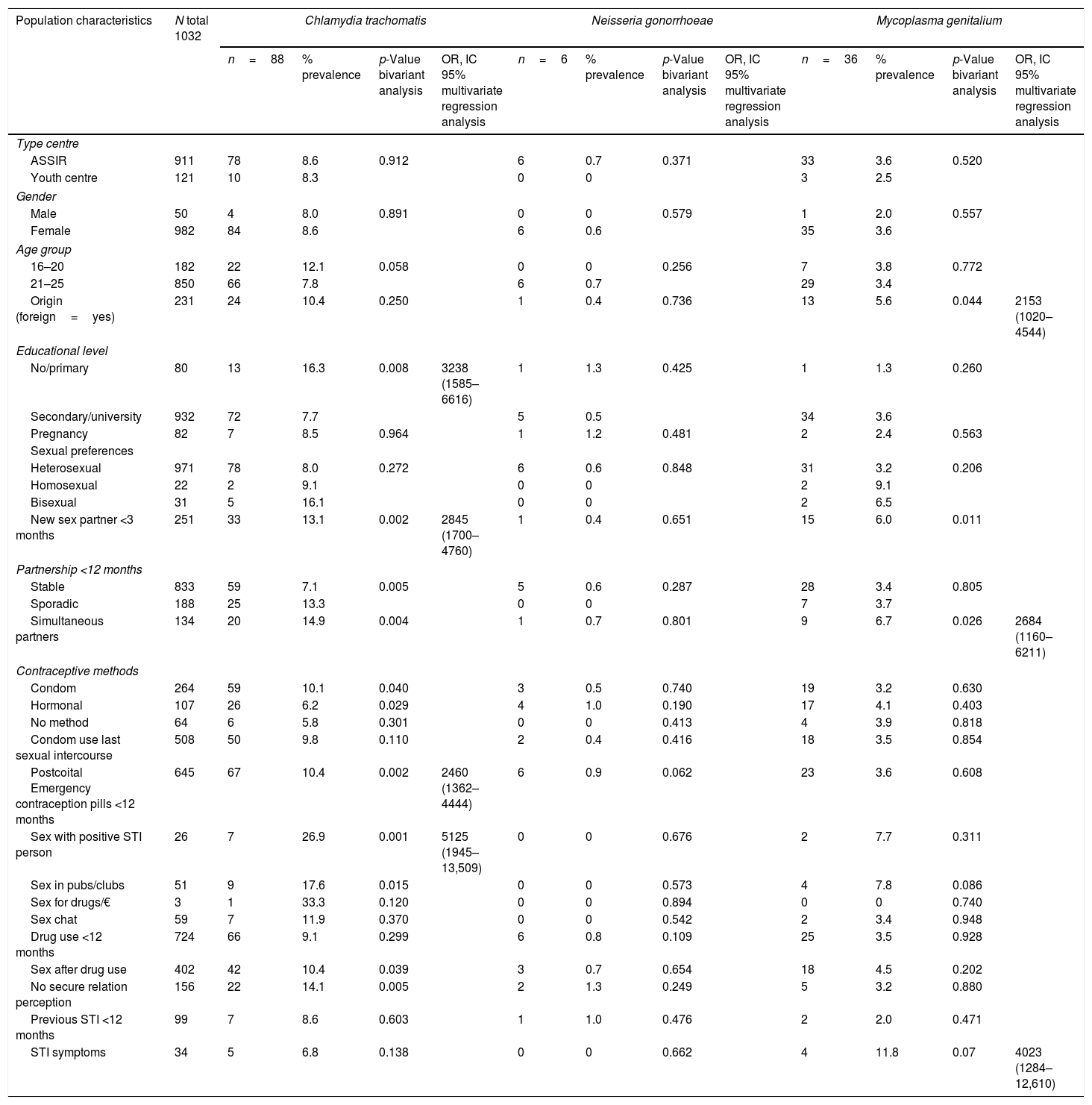
Population characteristicsA total of 1032 young <25 years from 24 ASSIR centres and three youth centres accepted to participate. The majority, 95%, were women with a mean age of 21.1 years and 22% foreign. Among women, 8.3% were pregnant at the moment of enrolment. Epidemiological characteristics of the participants are shown in Table 1.
Epidemiological characteristics, bivariate and multivariate regression analysis of Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium.
| Population characteristics | N total 1032 | Chlamydia trachomatis | Neisseria gonorrhoeae | Mycoplasma genitalium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=88 | % prevalence | p-Value bivariant analysis | OR, IC 95% multivariate regression analysis | n=6 | % prevalence | p-Value bivariant analysis | OR, IC 95% multivariate regression analysis | n=36 | % prevalence | p-Value bivariant analysis | OR, IC 95% multivariate regression analysis | ||
| Type centre | |||||||||||||
| ASSIR | 911 | 78 | 8.6 | 0.912 | 6 | 0.7 | 0.371 | 33 | 3.6 | 0.520 | |||
| Youth centre | 121 | 10 | 8.3 | 0 | 0 | 3 | 2.5 | ||||||
| Gender | |||||||||||||
| Male | 50 | 4 | 8.0 | 0.891 | 0 | 0 | 0.579 | 1 | 2.0 | 0.557 | |||
| Female | 982 | 84 | 8.6 | 6 | 0.6 | 35 | 3.6 | ||||||
| Age group | |||||||||||||
| 16–20 | 182 | 22 | 12.1 | 0.058 | 0 | 0 | 0.256 | 7 | 3.8 | 0.772 | |||
| 21–25 | 850 | 66 | 7.8 | 6 | 0.7 | 29 | 3.4 | ||||||
| Origin (foreign=yes) | 231 | 24 | 10.4 | 0.250 | 1 | 0.4 | 0.736 | 13 | 5.6 | 0.044 | 2153 (1020–4544) | ||
| Educational level | |||||||||||||
| No/primary | 80 | 13 | 16.3 | 0.008 | 3238 (1585–6616) | 1 | 1.3 | 0.425 | 1 | 1.3 | 0.260 | ||
| Secondary/university | 932 | 72 | 7.7 | 5 | 0.5 | 34 | 3.6 | ||||||
| Pregnancy | 82 | 7 | 8.5 | 0.964 | 1 | 1.2 | 0.481 | 2 | 2.4 | 0.563 | |||
| Sexual preferences | |||||||||||||
| Heterosexual | 971 | 78 | 8.0 | 0.272 | 6 | 0.6 | 0.848 | 31 | 3.2 | 0.206 | |||
| Homosexual | 22 | 2 | 9.1 | 0 | 0 | 2 | 9.1 | ||||||
| Bisexual | 31 | 5 | 16.1 | 0 | 0 | 2 | 6.5 | ||||||
| New sex partner <3 months | 251 | 33 | 13.1 | 0.002 | 2845 (1700–4760) | 1 | 0.4 | 0.651 | 15 | 6.0 | 0.011 | ||
| Partnership <12 months | |||||||||||||
| Stable | 833 | 59 | 7.1 | 0.005 | 5 | 0.6 | 0.287 | 28 | 3.4 | 0.805 | |||
| Sporadic | 188 | 25 | 13.3 | 0 | 0 | 7 | 3.7 | ||||||
| Simultaneous partners | 134 | 20 | 14.9 | 0.004 | 1 | 0.7 | 0.801 | 9 | 6.7 | 0.026 | 2684 (1160–6211) | ||
| Contraceptive methods | |||||||||||||
| Condom | 264 | 59 | 10.1 | 0.040 | 3 | 0.5 | 0.740 | 19 | 3.2 | 0.630 | |||
| Hormonal | 107 | 26 | 6.2 | 0.029 | 4 | 1.0 | 0.190 | 17 | 4.1 | 0.403 | |||
| No method | 64 | 6 | 5.8 | 0.301 | 0 | 0 | 0.413 | 4 | 3.9 | 0.818 | |||
| Condom use last sexual intercourse | 508 | 50 | 9.8 | 0.110 | 2 | 0.4 | 0.416 | 18 | 3.5 | 0.854 | |||
| Postcoital Emergency contraception pills <12 months | 645 | 67 | 10.4 | 0.002 | 2460 (1362–4444) | 6 | 0.9 | 0.062 | 23 | 3.6 | 0.608 | ||
| Sex with positive STI person | 26 | 7 | 26.9 | 0.001 | 5125 (1945–13,509) | 0 | 0 | 0.676 | 2 | 7.7 | 0.311 | ||
| Sex in pubs/clubs | 51 | 9 | 17.6 | 0.015 | 0 | 0 | 0.573 | 4 | 7.8 | 0.086 | |||
| Sex for drugs/€ | 3 | 1 | 33.3 | 0.120 | 0 | 0 | 0.894 | 0 | 0 | 0.740 | |||
| Sex chat | 59 | 7 | 11.9 | 0.370 | 0 | 0 | 0.542 | 2 | 3.4 | 0.948 | |||
| Drug use <12 months | 724 | 66 | 9.1 | 0.299 | 6 | 0.8 | 0.109 | 25 | 3.5 | 0.928 | |||
| Sex after drug use | 402 | 42 | 10.4 | 0.039 | 3 | 0.7 | 0.654 | 18 | 4.5 | 0.202 | |||
| No secure relation perception | 156 | 22 | 14.1 | 0.005 | 2 | 1.3 | 0.249 | 5 | 3.2 | 0.880 | |||
| Previous STI <12 months | 99 | 7 | 8.6 | 0.603 | 1 | 1.0 | 0.476 | 2 | 2.0 | 0.471 | |||
| STI symptoms | 34 | 5 | 6.8 | 0.138 | 0 | 0 | 0.662 | 4 | 11.8 | 0.07 | 4023 (1284–12,610) | ||
Target STI prevalences were: 8.5% C. trachomatis, 0.6% N. gonorrhoeae and 3.5% M. genitalium. The prevalence of Trichomonas vaginalis in positive pools of C. trachomatis, N. gonorrhoeae or M. genitalium was 0.8% (3/348).
Epidemiological characteristics are shown in Table 1; almost 95% of the C. trachomatis cases were women with a mean age of 21.5 years and 28% were foreign. The majority were heterosexual. In addition, they declared having an average of 2 sexual partners within the previous 12 months, 67% were stable partner and 37.8% declared a new sexual partnership ≤3 months. Condom use during last sexual intercourse declared in 56.8% and 76% use a postcoital emergency contraception pill (PECP). Five cases were symptomatic. Previous STI in 8%. Seven pregnant infected with C. trachomatis for a prevalence of 8.5% and all were asymptomatic. For N. gonorrhoeae, the majority were autochthonous with a mean age of 18 years and an average 2 sexual partners. All of them were heterosexual with 83% stable partner. Condom use during last sexual intercourse declared in 33.3% and all cases use PECP. No cases were symptomatic. Previous STI in 16.6%. One N. gonorrhoeae positive sample corresponded to asymptomatic pregnant for a prevalence of 1.2%. Most of the M. genitalium infected participants were women, older mean age 21 and 64% autochthonous. With an average of 2 sexual partners, 86.1% heterosexual, 77% with stable partner and 41.6% declared a new partner ≤3 months. Half of them declared condom use during last sexual intercourse and 64% use PECP. Four cases were symptomatic. Previous STI in 5.5%. Two M. genitalium positive samples corresponded to asymptomatic pregnant for a prevalence of 2.4%. The three positive cases for Trichomonas vaginalis were heterosexual women, 33.3% foreign and 66.7% with a stable partner. All cases declared a new sexual partner ≤3 months, condom use in the last sexual intercourse and use PECP.
After performing a multivariate logistic regression analysis for each STI, the factors associated with C. trachomatis were: low educational level, having a new sexual partner, use PECP and sex with positive STI-person. The factors associated with M. genitalium were foreign, simultaneity partnership and having symptoms. None risks factors were in N. gonorrhoeae (Table 1).
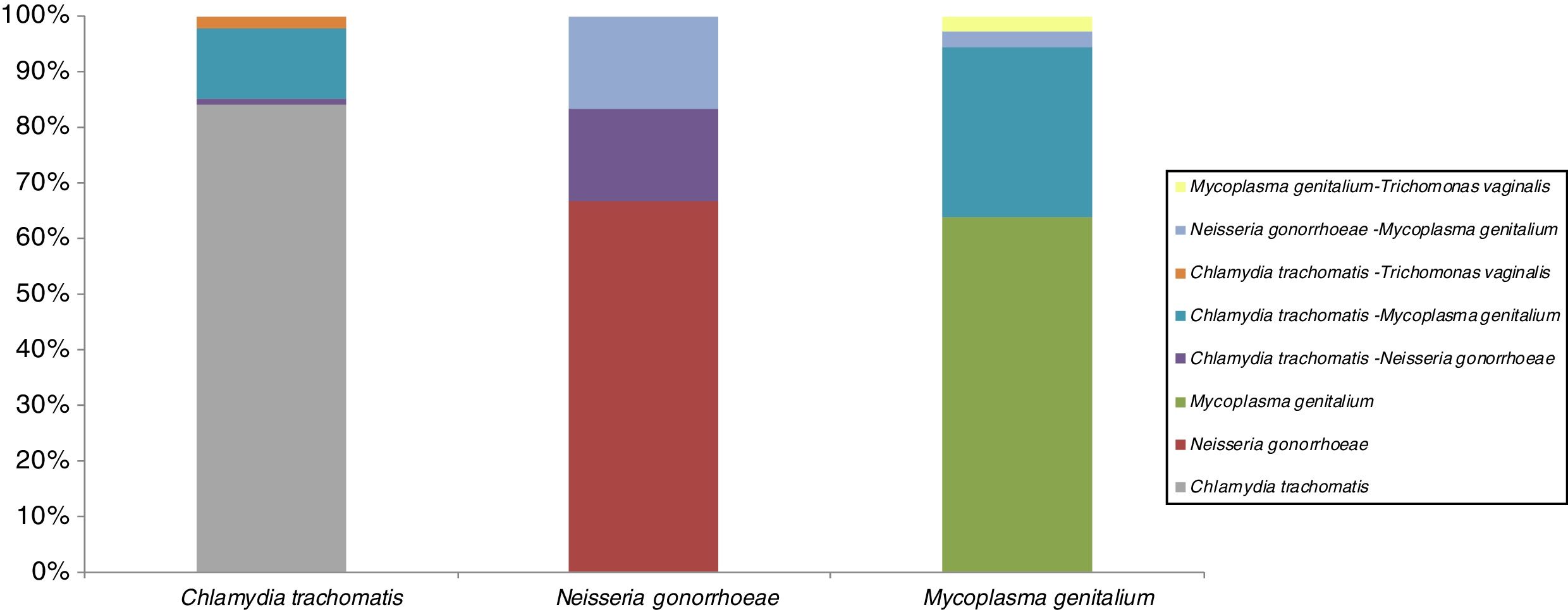
STI co-infectionsSTI co-infection combinations are shown in Fig. 1. All 3 targets STI in study are presented majority in single infection. The most prevalent single infection was C. trachomatis. This infection was co-infectivity with other microorganism in 16%: a 13% with M. genitalium, 2% with Trichomonas vaginalis and 1% with N. gonorrhoeae. About N. gonorrhoeae are presented in co-infection (34%) with the same proportion (17%) between C. trachomatis and M. genitalium. The 36% co-infected cases for M. genitalium are presented with 30% C. trachomatis and 3% both for N. gonorrhoeae and for Trichomonas vaginalis.
DiscussionThe present study, introduces for the first time in Catalonia a cost saving strategy for the STI prevalence and epidemiological monitoring of young population through the introduction of pooled urine specimens using NAAT's technology. The STI pooling strategy had been used in diverse scenarios as for opportunistic testing of C. trachomatis within a US Infertility Prevention Project including multiple STI measures using hierarchical pooling16 or for triple-site pooling among MSM17 or within asymptomatic pregnant women.18
Our findings showed savings cost of 33% as similar found by Peeling et al.9 when considering an initial 7% C. trachomatis prevalence at pool size of 3 specimens. Different costs reductions were found according to the pool size used to a specific prevalence. Shipitsyna et al. stated that cost-benefit depend on the prevalence of infection in the population and pooling size chosen.12 Peeling et al., compared cost savings using different pool sizes (2–5 specimens) at 5%, 10% and 20% C. trachomatis prevalence, obtaining savings of 31–39% (2–5 specimens/pool) at 10% prevalence and as high as 40–57% (2–5 specimens/pool) at 5% prevalence.9 Other study by Kucinskiene et al.19 obtained 7.1% C. trachomatis prevalence for the age group of 20–24 using three samples per pool and overall cost saving of 85%.
These results bring the need to perform further analysis increasing the number in each pool in order to evaluate a further increase in cost-savings within this population.
Our cost saving findings are consistent with other studies7,9,12,18 in a reduction between 30% and 50%. We analyzed saving costs based only in reagent costs and savings were calculated based on the percentage reduction in the number of tests associated with the pool strategy. The estimation of the full costs of the tests was not assessed because, tests kits cost and personnel cost varies according to the laboratory. In this way, the savings obtained have a wider applicability and comparability. This strategy has also been used by other authors.9 However, when cost of reagent, consumables and personnel were included into analysis, get a range of savings in reagents costs between 39% and 43%.20 The pooling strategy carried out in our context is a cost-saving strategy for epidemiological studies and screening programs as supported by Morré et al.21
The STI prevalence rates among the young population in our study are low except to C. trachomatis that continues on the rise. C. trachomatis prevalence was higher than the median prevalence observed in previous studies in Catalonia4 and by Fernández-Benítez et al. in Spain.22 In Catalonia the 10-year evolution of C. trachomatis showed a median prevalence of 7.7% and continues on the rise among young adults <25 years. The low prevalence obtained for N. gonorrhoeae was similar to those found in previous studies in our setting.4
For the first time, prevalence data of M. genitalium in Catalonia are showed. This prevalence is low in our study. In Europe prevalence range between 3% and 10%.23,24 In France, Pereyre et al.24 found comparable figures than ours within a similar population between 16 and 24 years (3.6%). On the contrary, in the USA the prevalence is higher. Getman et al.23 found 20.4% prevalence among young <30 years. Both studies shared similar settings and population. The only studied carried out in Spain by Asenjo et al.25 found prevalence of 3.3%, but in a symptomatic general population.
It has to be careful that with the prevalence rate found and the extensive testing, detection and subsequent antimicrobial treatment of M. genitalium performed in some settings may result in the selection of antimicrobial resistance punches, so there is a consensus of M. genitalium test in patients with non gonocal urethrithis, pelvic inflammatory disease or persistent vaginal discharge, so selective screening for M. genitalium in the general population may be a possibility in the future.26,27
Determinants associated with STI detected are similar than observed in previous studies.4,28 Use PECP appears for the first time as a C. trachomatis determinant in our studies. There is a concern that women who have easy access to a postcoital form of contraception may in fact have more unprotected intercourse and abandon more effective forms of regular contraception. Studies have also shown about the impact of increased access to PECP on sexual risk taking behaviours and STI29,30,31 and suggest that females who use PECP do not necessarily consider using a condom for STI prevention. In a large Canadian study, only 27% of current PECP users reported concurrent use of condoms.32
Our data are consistent from those reported previously23 in that testing for C. trachomatis and other sexually transmitted microorganisms using sensitive NAAT methods revealed that the majority of samples were infected with a single microorganism. This apparent predominance of subjects harbouring single-organism infections has implications for the diagnosis and treatment of individuals who are at high risk for STIs. Current treatment guidelines recommend antibiotic therapy that is specific to each microorganism33 and, since coinfection with one or more STI may be uncommon, the choice of therapy for managing mucosal epithelial inflammatory conditions should be guided by the employment of clinically validated diagnostic methods, rather than a syndromic management approach.
The limitations showed in our study are: (i) STI prevalence could be a slight underestimation given the pool strategy used because the effect of dilution could give a loss of sensitivity.9 (ii) Urine sample is probably not the most ideal sample in women and can give up to 10% of false negatives,34 but according to previous studies, non-invasive detection options, such as urine sample, could eliminate some of the barriers for the STI detection. Patients clearly prefer non-invasive methods and could substantially increase the acceptability and convenience of screening in a variety of settings. The easy collection and transport of urine samples makes the large-scale analysis of the selected populations feasible, especially considering that, in our context, young population is being analyzed and part of the study, which is carried out in non-health environment as youth care centres. In addition, current PCR techniques have high sensitivities and specificities in urine samples.9,35 (iii) The study sample was representative of the participating centres so the number of men is scarce but previous studies do not differ in C. trachomatis or M. genitalium prevalence according to sex.36,24 Regarding pregnancy, it is considered relevant to study this population more thoroughly, given that STI prevalence data in pregnant women in Spain is scarce, only Piñeiro et al.37 has carried out an analysis of these characteristics.
Despite these limitations, the present study introduces for the first time in Catalonia a cost saving strategy for STI prevalence and epidemiological monitoring of young population through the introduction of pooled urine specimens and it shows that the pooling carried out in our context is a cost-saving strategy for epidemiological studies and it can impact in the feasibility of STI screening programs. In the same way this study presents STI prevalence in young population, emphasizing that C. trachomatis prevalence continues to increase among this population and for the first time in Catalonia, are shown prevalence data of M. genitalium among young.
Conflicts of interestThe authors have no conflicts of interest to declare.
We acknowledge the work of investigators in the Study Group, collaborators from the Reproductive and Sexual Health Centres and the financial support of the Departament de Salut Generalitat de Catalunya and CIBER Epidemiología y Salud Pública (CIBERESP).
Nayade Crespo (ASSIR Bages-Bergueda-Solsones), M. Jose Garrofe, Rosa Alzuria, Elena Bureu, Isabel Fernandez, Montserrat Piquet, Yolanda Florensa, Carme Sarroca, Judit Mari, Elisabet Mitjans (ASSIR Lleida), Ivana Jorda (ASSIR Hospital Universitari Mutua Terrassa), Sonia Garcia (Consorci Hospital de Terrassa), Hildegard Mausbach, Teresa Gomez, Ainhoa Borras, Alba Llobera, Ana Estruch (ASSIR Mataro), Mabel Cayuela (Hospital de Terrassa), Josep Grau, Judit Tarres (ASSIR Osona), Dolors Guix, Lorena Serrano, Paula Amezcua, Meritxell Angelet, Carmen Barrionuevo, Rocio Calviño, Margarida Colldeforns, Mercè Duran, M. Jose Garcia, Maria Gonzalez, Gemma Hernandez, Jon Ander Modenes, Anna Vila (ASSIR Granollers), Carme Basset (ASSIR Cerdanyola), Angels Avecilla (ASSIR Badalona Serveis Assistencials), M. Pilar Blasco (ASSIR Sta. Coloma Gramenet), Lucia Burgos (ASSIR Mollet), Ramon Espelt, Edith Lopez-Grado (ASSIR Cerdanyola-Sabadell), M. Ines Molina (ASSIR Anoia-Igualada), Elisenda Prats (ASSIR Garraf), Demetria Patricio (ASSIR Reus), Gemma March, Irene Aguilar, Sonia Argiles, M. Jose Bayarri, Judith Bertran, Carmen Burgos, Montserrat Carreres, Ana Corredor, Victoria Fernandez, Gemma Martinez, Gracia Moreno, Alba Pallie, Teresa Pinto, Maria Ramirez, Cristina Rebollo, Eusebia Romano (ASSIR Tarragona-Valls), M. Consuelo Muxi (ASSIR Dreta), Rosa Escriche, Judit Pelegri, Luciano Carmelo Caccioppoli, Elisabet Grau, Jordi Xandri, M. Julia Cid, Laia Aguilar, Helena Almenar (ASSIR Esquerre), Carlos Navales (ASSIR Muntanya), Jordi Baroja, M. Paz Oliver (Centre jove d’anticoncepció sexualitat, CJAS Bcn), Carme Fornells, Marta Hernandez (Centre jove de salut de Girona), Estrella Arranz (Punt de Salut Jove Hospitalet).