Current gold standard diagnostic techniques for dengue are expensive and time-consuming. Rapid diagnostic tests (RDTs) have been proposed as alternatives, although data about their potential impact in non-endemic areas is scarce.
MethodsWe performed a cost-effectiveness analysis comparing the costs of dengue RDTs to the current standard of care for the management of febrile returning travelers in Spain. Effectiveness was measured in terms of potential averted hospital admissions and reduction of empirical antibiotics, based on 2015–2020 dengue admissions at Hospital Clinic Barcelona (Spain).
ResultsDengue RDTs were associated with 53.6% (95% CI: 33.9–72.5) reduction of hospital admissions and were estimated to save 289.08–389.31€ per traveler tested. Moreover, RDTs would have avoided the use of antibiotics in 46.4% (95% CI: 27.5–66.1) of dengue patients.
DiscussionImplementation of dengue RDTs for the management of febrile travelers is a cost-saving strategy that would lead to a reduction of half of dengue admissions and a reduction of inappropriate antibiotics in Spain.
El actual gold standard para el diagnóstico de dengue se basa en técnicas caras y que requieren tiempo. Los tests de diagnóstico rápido (TDR) se han propuesto como una posible alternativa, aunque los datos sobre su posible impacto en áreas no endémicas son escasos.
MétodosRealizamos un análisis de coste-efectividad comparando los costes del uso de TDR para dengue con el manejo habitual de viajeros con fiebre en España. Para medir la efectividad se estimaron las hospitalizaciones potencialmente evitables y la reducción de antibióticos empíricos de acuerdo con las hospitalizaciones por dengue entre 2015-2020 en el Hospital Clínic Barcelona (España).
ResultadosEl uso de TDR para dengue se asoció con una reducción de 53.6% (IC 95%: 33.9–72.5) de las hospitalizaciones y un ahorro de 289.08-389.31€ por viajero testado. Además, el uso de TDR hubiese evitado el tratamiento de antibióticos en 46.4% (IC 95%: 27.5–66.1) de los casos de dengue.
DiscusiónLa implementación de TDR de dengue para el manejo de viajeros con fiebre es una medida de reducción de gastos que disminuiría a la mitad los ingresos hospitalarios por dengue y supondría una reducción del uso inapropiado de antibióticos en España.
Dengue is the most common arboviral infection worldwide, with about half of the world's population at risk of being infected, and an 8-fold increase in the number of cases over the last two decades.1 The increasing incidence of dengue worldwide and the occurrence of outbreaks in endemic-areas also affects travelers from non-endemic areas, making dengue the main cause of fever in returning travelers with acute undifferentiated fever.2
The gold standard for dengue diagnosis is based on nucleic acid amplification tests (NAAT) and antibody detection by serology tests.3 However, these tests are expensive and seldom available in most health-care facilities, and they are only routinely performed in reference microbiology laboratories. Moreover, because laboratory results are not immediately available, unnecessary admissions and antibiotic prescriptions may occur.
Antigen detection by rapid diagnostic tests (RDT) has been proposed as a potential alternative which could better guide patients’ management thanks to the possibility of getting a quick and inexpensive diagnosis.3,4 However, dengue RDTs usefulness in endemic areas has been questioned because of their lower sensitivity and specificity in secondary dengue cases and the lack of conclusive results in cost-effectiveness studies, but data about non-endemic areas is scarce.4,5
With this study, we aimed to evaluate the potential impact on hospital admissions, healthcare costs and empirical antibiotic prescriptions of including dengue RDTs for the management of febrile travelers in Spain.
Material and methodsThe study was conducted from the health care system perspective and evaluated the potential impact of dengue RDT on three different outcomes: (i) hospital admissions in dengue patients, (ii) the healthcare costs of providing medical assistance to travelers with undifferentiated non-malarial fever (UNMF), and (iii) the use of empirical antibiotics in hospitalized dengue patients in Spain.
Estimates of the potentially averted hospital admissions were based on adult dengue admission records at Hospital Clinic in Barcelona, a reference hospital for tropical medicine and imported diseases in Spain, from 2015 to 2020. Cases presenting with no criteria for severe dengue, no warning signs and no other clinical criteria for admission were considered as admissions that could have been averted.
A decision-tree model was developed to estimate the healthcare costs associated with the use of dengue RDTs for the management of adult travelers with UNMF. Three different dengue RDTs (anonymized as Test A, Test B and Test C) that had been already tested in non-endemic settings were considered.6–8 RDT costs were compared to the costs of using dengue NAAT and serology, which is the gold standard for the diagnosis of dengue and the current practice at Hospital Clínic in Barcelona3,9 (Supplementary figure). After considering RDTs diagnostic performance, hospital admission rates were applied to the following subgroup of patients: true positives, false negatives, false positives, true negatives, and untested patients (Supplementary Table 1). We considered the costs of outpatient care in all patients, and the costs of the tests included in the diagnostic work-up of UNMF in patients with a negative or no dengue RDT.
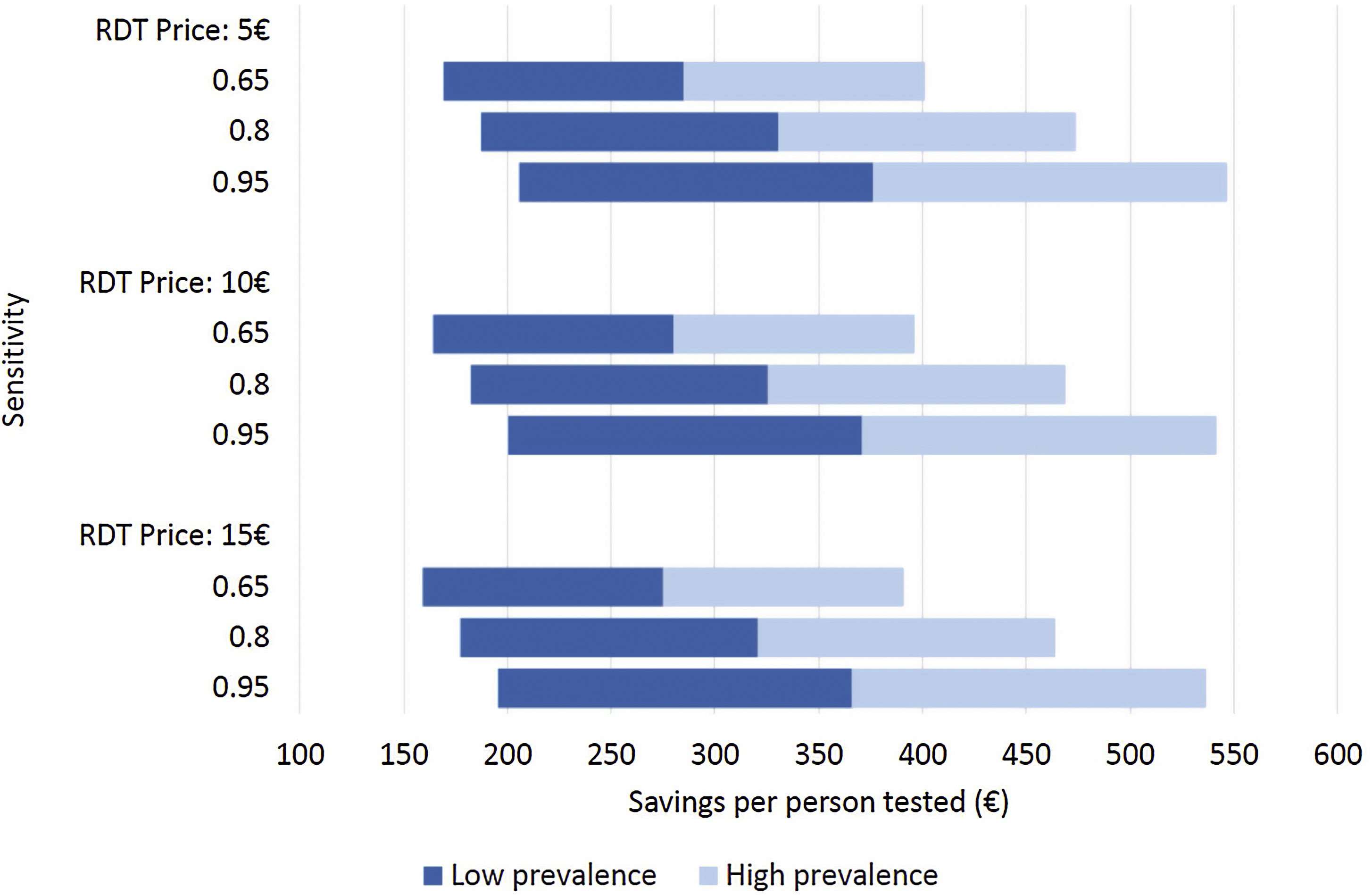
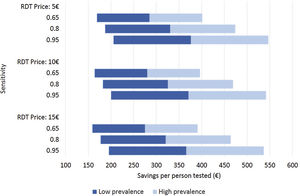
Costs of dengue RDTs were based on published literature, integrated by information provided by the manufacturer.6,7,10 The costs falling on the health care system were calculated by multiplying the number of hospital admissions with an estimate of an average cost per admission episode. Information on dengue admission rates and health care costs were obtained from the Spanish Hospital Discharge Records Database (CMBD) and the Dengue Annual Epidemiological Reports from European Centre for Disease Prevention and Control (ECDC).11 Dengue prevalence and admission rate for UNMF were taken from a recent European multicenter study that investigated causes of fever in travelers.2 Costs are presented in 2022 euro (€). No discount rate was considered because time horizon considered was shorter than 1 year. Appropriate distributions were assigned to the relevant parameters and a probabilistic sensitivity analysis based on 1000 Monte Carlo iterations was conducted (Supplementary Table 1). To assess the robustness of the results to changes in the model assumptions, different scenarios were explored (Fig. 1).
Different scenarios of dengue rapid-diagnostic tests (RDT) in travelers with undifferentiated non-malarial fevers (UNMF). Scenarios analysis considering: (i) RDT price of 5€, 10€ and 15€; (ii) dengue RDT sensitivity of 0.65, 0.8 and 0.95; and (iii) dengue prevalence among travelers with UNMF of 0.25, ranging from 0.1 (lower prevalence) to 0.4 (higher prevalence). X-axis represents the estimated savings per patient with UNMF tested (€).
Additionally, the potential impact of using dengue RDTs for the management of travelers with fever was also measured by the potential reduction in empirical antibiotic use in hospitalized dengue patients through the retrospective review of dengue admission records at Hospital Clinic in Barcelona from 2015 to 2020. Empirical antibiotics prescribed to patients finally diagnosed with dengue, without microbiologically confirmed bacterial infections and without clinical signs or symptoms of bacterial infections were classified as antibiotic treatments that could have been averted.
Results were reported following the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022)12 (Supplementary Table 2). The study was designed in compliance with Good Clinical Practice and following the Declaration of Helsinki. The Ethics Committee at Hospital Clinic of Barcelona approved the study.
ResultsAmong the 289 dengue cases diagnosed during the study period (2015–2020) at Hospital Clinic of Barcelona, 28 (9.7%) were admitted to hospital. Of these, none was diagnosed with severe dengue, 13 (46.4%) presented warning signs (n=10) or other clinical criteria for admission (n=3). The remaining 15 patients were admitted to hospital to complete the diagnostic workup of UNMF. The introduction of dengue RDTs would have hence averted 53.6% (95% CI: 33.9–72.5) of dengue admissions.
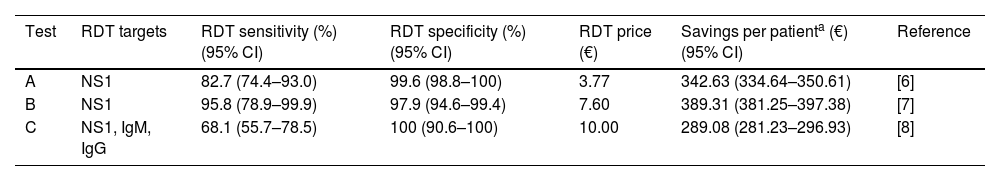
As a result, cost savings associated with the different RDTs ranged from a minimum of 289.08€ (95% CI: 281.23–296.93) to a maximum of 389.31€ (95% CI: 381.25–397.38) for each traveler with UNMF tested with a dengue RDT (Table 1). The introduction of RDTs appeared to be associated with cost savings in all scenarios considered. Even in the most pessimistic scenario, with a relatively costly (15€) and low sensitive (0.65) RDT used in a context of low dengue prevalence (0.10), its implementation would save 159.14€ per tested patient. Savings would increase up to 546.63€ per patient in the most optimistic scenario, considering an inexpensive (5€) RDT with high sensitivity (0.95), in a context of high dengue prevalence (0.4) (Fig. 1).
Probabilistic cost analysis of dengue rapid-diagnostic tests (RDT) in travelers with undifferentiated non-malarial fevers (UNMF).
| Test | RDT targets | RDT sensitivity (%) (95% CI) | RDT specificity (%) (95% CI) | RDT price (€) | Savings per patienta (€) (95% CI) | Reference |
|---|---|---|---|---|---|---|
| A | NS1 | 82.7 (74.4–93.0) | 99.6 (98.8–100) | 3.77 | 342.63 (334.64–350.61) | [6] |
| B | NS1 | 95.8 (78.9–99.9) | 97.9 (94.6–99.4) | 7.60 | 389.31 (381.25–397.38) | [7] |
| C | NS1, IgM, IgG | 68.1 (55.7–78.5) | 100 (90.6–100) | 10.00 | 289.08 (281.23–296.93) | [8] |
According to Hospital Clinic of Barcelona cases, empirical antibiotics were prescribed in 15 (53.6%) of the 28 hospitalized patients finally diagnosed with dengue. However, 13 (86.7%) of them did not present microbiological evidence nor signs or symptoms of bacterial infections. Therefore, 46.4% (95% CI: 27.5–66.1) of admitted dengue received unnecessary antibiotics for an average duration of 6.1 days per patient.
DiscussionAccording to our results, implementation of dengue RDTs for the management of returning travelers with UNMF in Spain could reduce at least 50% of dengue hospital admissions and hence save from 289 to 389€ per each traveler tested. Further, it could also help to avoid unnecessary antibiotics in almost half of admitted dengue patients.
Therefore, the inclusion of dengue RDTs in clinical practice would not only improve the management of patients with imported fever, by allowing a prompt diagnosis of dengue and avoiding unnecessary antibiotics and hospitalizations, but also reduce the economic burden of dengue in non-endemic areas. This is particularly relevant given the increasing incidence of dengue infection among international travelers during the last decades.2
The results of our study are based on conservative assumptions and estimates. First, the proportion of potentially avoidable dengue admissions was estimated from the records of a referral center for imported diseases. According to the CMBD and ECDC annual reports, the current global admission rates for dengue in Spain is approximately 32.8%. The 9.7% rate of dengue hospitalization adopted in our study is therefore significantly lower than the national average. These differences may due to the fact that febrile travelers attending hospitals with less experience in the management of imported fever are more likely to be admitted. Consequently, if RDTs were implemented at a national level the number of dengue admissions potentially avoidable might be three times higher.
Second, empirical antibiotics could be prescribed also in outpatient visits. However, prescriptions for outpatient dengue cases were not considered in the analysis. Therefore, the reduction in empirical antibiotic may have been underestimated. Nevertheless, our results still indicate that the adoption of dengue RDTs could contribute to antibiotic stewardship policies. Our findings reflect previous published cost-effectiveness analyses, where dengue RDT resulted in a reduction in antibiotics prescription compared to current management of acute febrile illnesses.13
Third, because the delay in the notification of imported dengue cases is the major risk factor for secondary autochthonous dengue cases in non-endemic areas,14 an early detection of dengue cases would trigger a prompt an early response from Public Health Agencies and improve the performance of Aedes spp. control strategies. Therefore, the implementation of dengue RDTs could not only improve the management of dengue cases and reduce healthcare costs, but also decrease the risk of introduction dengue virus to non-endemic areas where Aedes spp. is established, such as Spain.3,15,16 This is particularly relevant since several autochthonous dengue cases have already been reported in the Mediterranean region during the last years.16,17
To assess the robustness of our results, we investigated alternative scenarios. In the most pessimistic scenario analysis, we included very conservative assumptions such as a 10% prevalence of dengue and RDT costs above the current market prices. It was found that even with an expensive (15€) and non-sensitive (0.65) RDT in a low dengue prevalence context (0.10), the adoption of RDTs was associated to cost savings of over 150€ per tested patient. These results appear to be in contrast with previous studies conducted in endemic areas, where dengue RDTs were associated with higher costs compared to the standard of care for the management of patients with acute febrile illnesses.13 Nevertheless, such discrepancies could be due to differences in hospitalization costs and in the proportion of secondary dengue cases, which have lower RDTs sensitivities and higher proportion of severe cases.3,5,6
Finally, implementation of dengue RDTs would be beneficial not only in high-specialized tertiary hospitals, but also in less specialized healthcare settings, potentially with less travelers admitted to emergency rooms and no routinely access to standard diagnostic techniques for dengue. Although a low prevalence of dengue patients and the potential overuse of dengue RDTs could reduce the positive impact of introducing them, we estimated that even in very low prevalence scenarios (only 1 dengue case for each 10 travelers tested), the implementation of dengue RDTs would lead to cost-savings. Moreover, hospitalization rates of travelers with dengue are likely to be higher in non-specialized setting, where costs of providing standard of care are also likely to be higher, due to accessibility issues (e.g. transportation of samples to reference laboratories). Such constraints may also increase the time to obtain a diagnosis, thereby prolonging admission time, increasing the number of diagnostic tests performed and the prescription of empirical antibiotics. Consequently, our study supports the implementation of RDTs even in less specialized healthcare settings and in areas with fewer patients presenting fever after an international travel.
In conclusion, the implementation of dengue RDTs for the management of febrile travelers in Spain appears to be a cost-saving strategy that would lead to a reduction of half of dengue admissions and a reduction of inappropriate antibiotic use, with savings of 289–389€ per patient with UNMF tested. The use of dengue RDT should be included in clinical guidelines for the management of imported fever in Spain.
Authors contributionsDCF and JM conceptualized the study. DCF designed the study, wrote the main manuscript text and prepared tables and figures. LBS and AG collected the data. DCF, FR and ES performed the analysis. All authors reviewed the manuscript.
FundingThis work was not supported by any source of funding.
Conflicts of interestThe authors declare having no conflicts of interest.
We would like to thank Alejandra Gimenez for their help obtaining some economic data for the study, and Claudio Parolo for the continuous discussions about RDTs, which led to the generation of some of the research questions of this article. Finally, we would like to thank Mikel J. Martinez for his lab work on the routine diagnosis of arboviral infections, as well as all the physicians and nurses from the Department of International Health at the Hospital Clinic of Barcelona, who participated in the diagnosed and treatment of the dengue cases presented.