An analysis was made about the evolution of resistance to 3rd generation cephalosporins, imipenem, and other antibiotics in invasive isolates of Klebsiella pneumoniae according to the Spanish EARS-Net database (2010–2014).
MethodsForty-two hospitals from 16 Autonomous Communities with an approximate population coverage of 33% participated.
ResultsA total 7140 pneumoniae corresponding to the same number of patients were studied. Overall resistance percentages (I+R) were: cefotaxime 15.8%, ceftazidime 13.7%, imipenem 1.7%, ciprofloxacin 20.1%, tobramycin 14.1%, gentamicin 10.4%, and amikacin 1.9%.
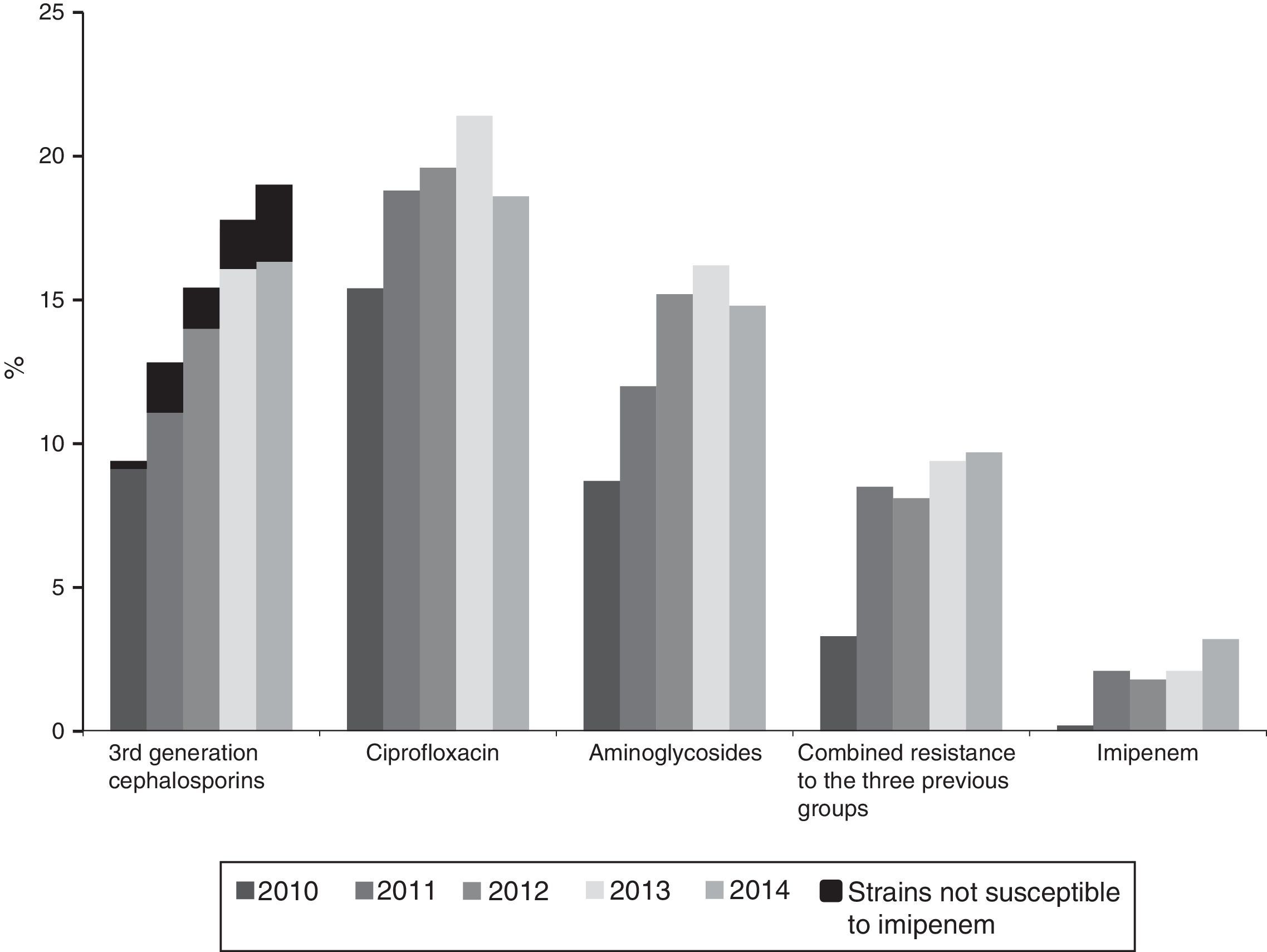
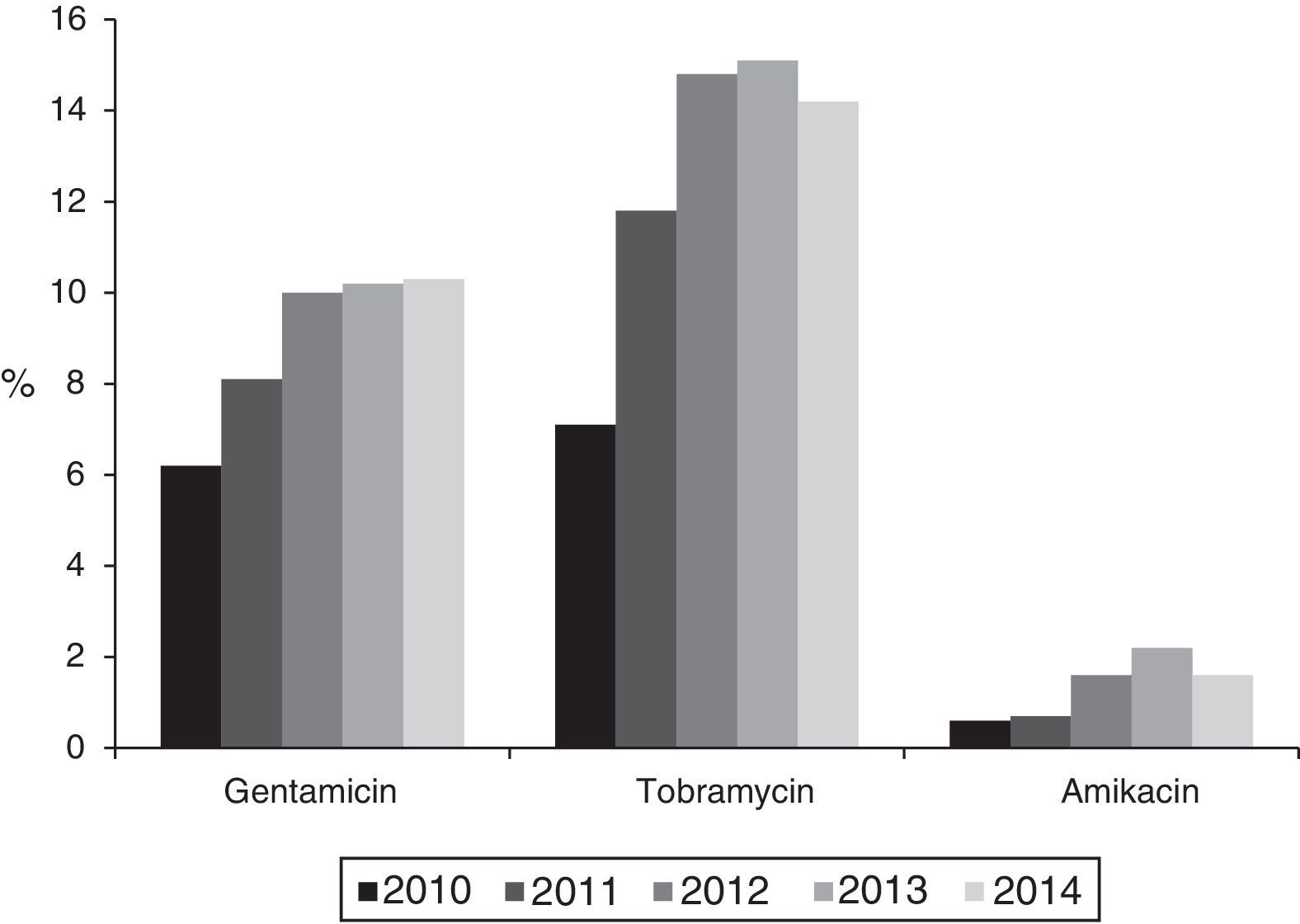
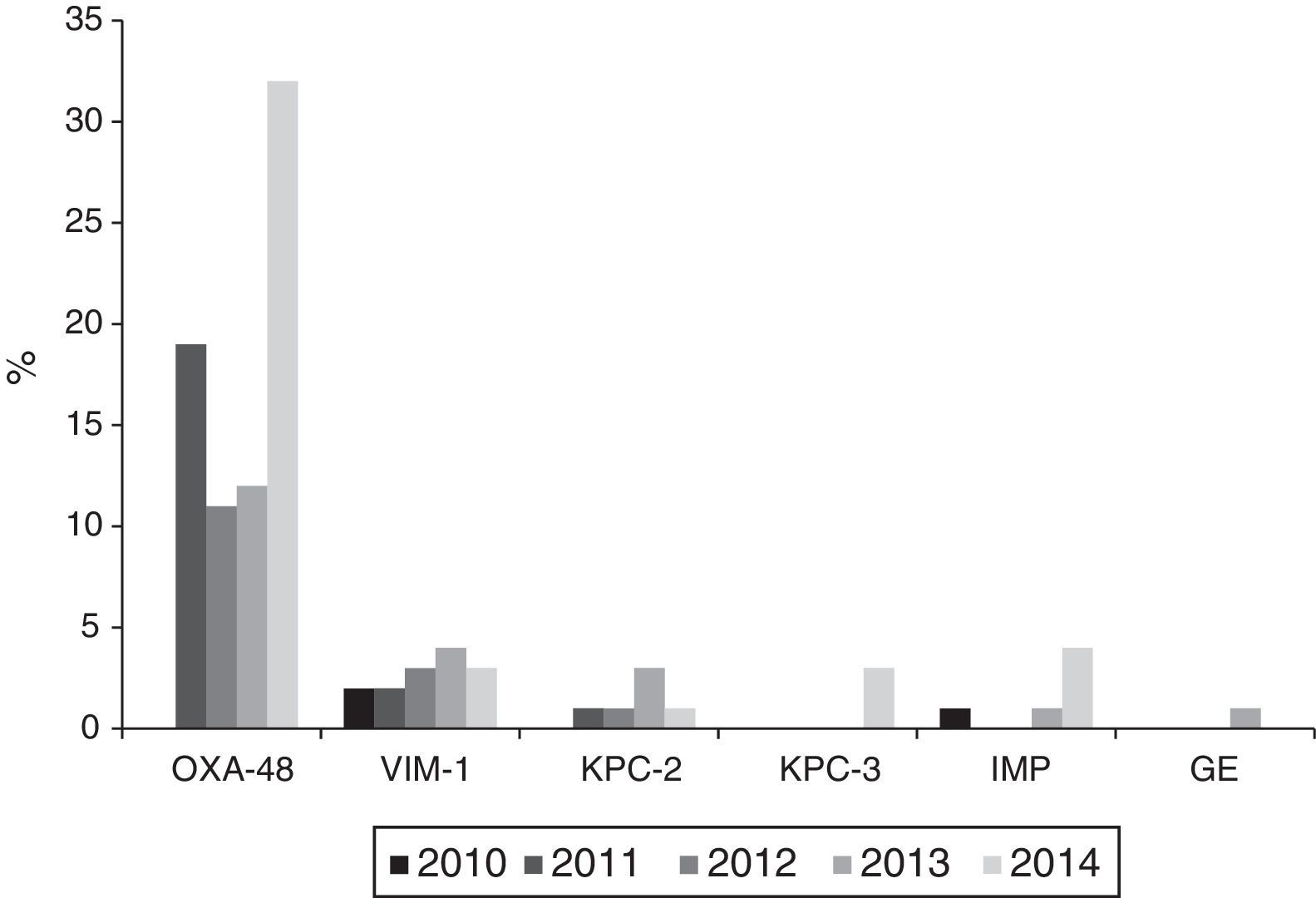
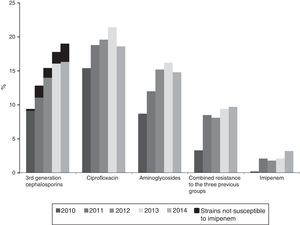
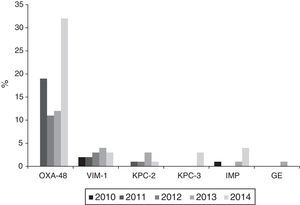
Resistance to 3rd generation cephalosporins increased from 9.8% (2010) to 19% (2014); to ciprofloxacin from 15.4% (2010) to 19.6% (2014); to gentamicin from 6.2% (2010) to 10.3% (2014) and to tobramycin from 7.1% (2010) to 14.2% (2014) (p<0.001 in all cases). Combined resistance to 3rd generation cephalosporins, ciprofloxacin, and aminoglycosides increased from 3.3% (2010) to 9.7% (2014) (p<0.001). Resistance to imipenem also increased from 0.27% (2010) to 3.46% (2014) (p<0.001). A total of 121 isolates were resistant to imipenem, of which 104 (86%) produced carbapenemases: 74 OXA-48, 14 VIM, 9 KPC (6 KPC-2 and 3 KPC-3), 6 IMP, and 1 GES.
ConclusionOver the 5 year period (2010–2014), resistance to 3rd generation cephalosporins in invasive K. pneumoniae in Spain has doubled. The combined resistance to 3rd generation cephalosporins, ciprofloxacin, and aminoglycosides has tripled, and imipenem resistance has increased almost 13 times, mostly due to the spread of carbapenemase-producing isolates.
Se analizó la evolución de la resistencia a cefalosporinas de 3.a generación, imipenem y otros antibióticos en aislamientos invasivos de Klebsiella pneumoniae (K. pneumoniae) según resultados de EARS-Net entre 2010 y 2014 en España.
MétodosParticiparon 42 hospitales de 16 Comunidades Autónomas, con una cobertura poblacional aproximada del 33%.
ResultadosSe aislaron 7.140 cepas de K. pneumoniae de un mismo número de pacientes. Las resistencias globales (I+R) fueron: cefotaxima 15,8%, ceftazidima 13,7%, imipenem 1,7%, ciprofloxacina 20,1%, tobramicina 14,1%, gentamicina 10,4% y amikacina 1,9%.
La resistencia a cefalosporinas de 3.a generación aumentó desde el 9,8% (2010) al 19% (2014); la de ciprofloxacina desde el 15,4% (2010) al 19,6% (2014); la de gentamicina desde el 6,2% (2010) al 10,3% (2014) y la de tobramicina desde el 7,1% (2010) al 14,2% (2014) (p<0,001 en todos los casos). Las cepas resistentes a la vez a cefalosporinas de 3.a generación, ciprofloxacina y aminoglucósidos aumentaron desde el 3,3% (2010) al 9,7% (2014) (p<0,001). La resistencia a imipenem aumentó desde el 0,27% (2010) al 3,46% (2014) (p< 0,001); 121 aislados fueron resistentes a imipenem, de los cuales 104 (86%) produjeron carbapenemasas: 74 OXA-48, 14 VIM, 9 KPC (6 KPC-2 y 3 KPC-3), 6 IMP y 1 GES.
ConclusionesEn un periodo de 5 años (2010-2014), la resistencia a cefalosporinas de 3.a generación en K. pneumoniae invasivas en España se ha duplicado; la resistencia combinada a cefalosporinas de 3.a generación, ciprofloxacina y aminoglucósidos se ha triplicado; la resistencia a imipenem ha aumentado casi 13 veces, principalmente por la diseminación de aislados productores de carbapenemasas.
Resistance to antibiotics constitutes one of the main threats to public health and to the individual health of patients around the world (1–3). The top priority in this situation is monitoring resistant pathogens with a high clinical and epidemiological impact, which are able to cause outbreaks and epidemics and for which there are few therapeutic options.1–3
Klebsiella pneumoniae is one of these pathogens. It is able to cause significant nosocomial outbreaks in high-risk units such as ICUs, onco-haematology or neonatology.4 The main therapeutic options for treatment of invasive K. pneumoniae infections include 3rd generation cephalosporins (cefotaxime, ceftazidime); however, the recent spread of K. pneumoniae strains that are resistant to these antibiotics has generated a significant clinical and epidemiological problem because: (i) the therapeutic options are reduced due to co-resistance; (ii) the consumption of carbapenem antibiotics considered last-line antibiotics, i.e., those used when the bacteria is resistant to the rest of antibiotics, increases.
EARS-Net is the official European surveillance network for antibiotic resistance in invasive pathogens. Coordinated by the European Centre for Disease Prevention and Control (EDEC, http://ecdc.europa.eu/en/Pages/home.aspx) since 2010, it currently collects information from 31 countries, 460 laboratories and 1300 hospitals.5 The surveillance system is continuous, not based on prevalence thresholds.
EARS-Net is a network made up of all the national networks established in the different European countries. The Spanish network operates in accordance with the general EARS-Net recommendations and is made up of a network of sentinel hospitals, representing the Spanish geography, that collect data about invasive infections (blood and CSF) caused by bacteria with a high clinical impact and a great ability to develop antibiotic resistance. Said information is sent quarterly to the Centro Nacional de Microbiología (CNM) of the Instituto de Salud Carlos III (ISCIII) for analysis and subsequent submission to the ECDC. The pathogens currently subjected to surveillance by EARS-Net are: Escherichia coli, K. pneumoniae, Pseudomonas aeruginosa, Acinetobacter ssp., Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis and Enterococcus faecium. The population coverage of EARS-Net in Spain is 14,600,000 inhabitants, around 33% of the population in 2014, and in Europe it is around 100 million people.
The objective of this study is to analyse the evolution of resistance to 3rd generation cephalosporins and other antibiotics, in K. pneumoniae haemoculture isolates over a 5-year period (2010–2014), according to data collected by the EARS-Net in Spain. Furthermore, in isolates with reduced susceptibility to imipenem (resistant and intermediate), the production of carbapenemases and the class to which they belonged were identified.
Materials and methodsAll the K. pneumoniae isolates collected from blood from Spanish hospitals participating in the EARS-Net from 2010–2014 were analysed. 42 hospitals belonging to 16 Autonomous Regions participated; 38 (90.5%) of them (15 Autonomous Regions) provided information during all five years. Only the first isolate per patient and year was included.
The participating hospitals were: 2 primary, 12 secondary and 28 tertiary. Fifteen hospitals had between 200 and 500 beds, 20 between 500 and 1000 beds and 7 had more than 1000. Two tertiary hospitals collected data from other associated hospitals with fewer than 100 beds.
The antibiotics subjected to vigilance in K. pneumoniae are: cefotaxime, ceftazidime, gentamicin, tobramycin, amikacin, ciprofloxacin and imipenem. EARS-Net Spain also includes: amoxicillin-clavulanic acid, piperacillin-tazobactam and co-trimoxazole.
The collection and analysis of antibiotic susceptibility data is done according to the general EARS-Net procedures (http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Documents/2015-EARS-Net-reporting-protocol.pdf). Each laboratory determined the antibiotic susceptibility and interpreted its results with the interpretation methods and criteria that it normally uses. K. pneumoniae susceptibility testing was conducted in all cases using commercial microdilution. The data was submitted prospectively to the CNM of the Instituto de Salud Carlos III where it was added to a common database using the free-to-use application of the Whonet program from the World Health Organization6; also, the results were validated and analysed according to the utilities for such purpose in said program. The susceptibility results were interpreted according to the Clinical Laboratory Standards Institute (CLSI) criteria.7 The European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria were not generally applied due to the fact that some of the commercial systems used by the participating hospitals during the study period did not include an adequate concentration range for their application. However, they were used to conduct a comparative analysis of the evolution of resistance to 3rd generation cephalosporins.8,9
In this paper, reduced susceptibility to an antibiotic was considered as the absence of susceptibility (intermediate susceptibility and resistance) to said antibiotic.
During the five-year period of the study, all participating laboratories underwent an external quality control organised annually by NEQAS (http://www.ukneqasmicro.org.uk/).
Although the information is not routinely recorded by EARS-Net, in isolates with reduced susceptibility to imipenem (MIC>1mg/l) the production of carbapenemases was studied and, in positive cases, the class of carbapenemases was identified using the previously-described phenotypic and genotypic methods.10
The comparison of prevalence of resistance to antibiotics between the various groups and the measures of association were calculated using the χ2 applied test of evolutionary trends, with a confidence interval (CI) of 95%. In all comparisons, the null hypothesis was rejected with a p≤0.05. The statistical analysis was conducted with the GraphPad Prism 6 software (Graph Pad Prism Software Inc., 7825 Fay Avenue, Suite 230, La Jolla, CA 92037, USA).
ResultsGeneral resultsBetween 2010 and 2014, EARS-Net Spain recorded a total of 7140 K. pneumoniae isolates from haemocultures (annual mean: 1428; range: 1198–1604; median: 1161). The annual distribution was: 1198 isolates in 2010, 1251 in 2011, 1509 in 2012, 1578 in 2013 and 1604 in 2014. The increase in isolates in the last two years was due to the incorporation of two large university hospitals that provided a high number of isolates.
Globally, the age distribution was: 534 (7.5%) isolates were obtained from patients aged 0–14 years; 2545 (35.6%) in patients aged 15–64 years and 4061 (56.9%) in patients aged 65 years or older. For hospital services, 2579 (36.1%) isolates came from medical departments, 1880 (26.3%) from emergency departments, 1238 (17.3%) from ICUs, 839 (11.8%) from surgical departments and 604 (8.5%) from other departments.
Resistance to β-lactam antibiotics in the 7140 isolates were: amoxicillin-clavulanic acid 23.3%, cefotaxime 15.8%, ceftazidime 13.7%, piperacillin-tazobactam 13.1% and imipenem 1.7%.
The non β-lactam antibiotic with greatest resistance was co-trimoxazole (22.4%), followed by ciprofloxacin (20.1%), tobramycin (14.1%), gentamicin (10.4%) and amikacin (1.9%).
Temporary evolution of resistanceThe analysis of evolutionary trends of antibiotic resistance and all subsequent analyses were calculated only based on the results of the 38 hospitals that participated during the entire study period; 6076 isolates of K. pneumoniae were studied between 2010 and 2014 (annual mean: 1215.2; range: 1072–1416); of which, 1072 were isolated in 2010, 1047 in 2011, 1215 in 2012, 1326 in 2013 and 1416 in 2014.
By age, 421 isolates (6.9%) came from patients 0 to 14 years of age, 1864 (30.7%) from patients aged 15 to 64 years and 3791 (62.4%) from those aged 65 years or older. For hospital services, 2189 isolates (36%) came from medical departments, 1621 (26.7%) from emergency departments, 1055 (17.3%) from ICUs, 736 (12.1%) from surgical departments and 475 (7.8%) from other departments.
The annual evolution of antibiotic resistance according to CLSI criteria are shown in Figs. 1 and 2. The percentage of resistance to 3rd generation cephalosporins increased from 9.8% (95% CI: 8.0–11.6%) (101 isolates with reduced susceptibility) in 2010 to 19% (95% CI: 16.9–21.1%) in 2014 (269 isolates) (χ2 of the trend: 50.5; p<0.0001) (Fig. 1). This evolutionary trend was also observed when separately studying cefotaxime and ceftazidime as follows: from 9.8% (95% CI: 8.0–11.6%) to 19.4% (95% CI: 17.3–21.5%) for cefotaxime (χ2 of the trend: 50.9; p<0.0001) and of 8.4% (95% CI: 6.8–10%) to 19% (95% CI: 16.9–21.1%) (χ2 of the trend: 46; p<0.0001) for ceftazidime. The significant increase of the resistance detected with CLSI criteria was also maintained upon calculating the trend using the EUCAST recommended cut-off values; the resistance to 3rd generation cephalosporins according to EUCAST criteria, increased from 10.2% in 2010 to 20% in 2014 (χ2 of the trend: 47; p<0.0001).
Resistance to ciprofloxacin increased from 15.4% (95% CI: 13.3–17.5%) in 2010 (138 isolates with reduced susceptibility) to 19.6% (95% CI: 17.5–21.7%) in 2014 (263 isolates) (χ2 of the trend: 13.3; p: 0.0002) (Fig. 1).
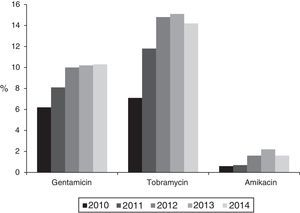
The percentage of isolates with reduced susceptibility to at least one of the tested aminoglycoside antibiotics (gentamicin, tobramycin and amikacin) increased from 8.8% (95% CI: 7.2–10.4%) to 14.8% (95% CI: 12.8–16.8%) between 2010 and 2014 (χ2 of the trend: 24.9; p<0.0001) (Fig. 1). Analysed individually, the greatest increase was detected in resistance to tobramycin, which increased from 7.1% (95% CI: 5.9–8.6%) in 2010 (76 isolates with reduced susceptibility) to 14.2% (95% CI: 12.2–16.2%) in 2014 (201 isolates) (χ2 of the trend: 49; p<0.0001). For its part, resistance to gentamicin increased from 6.2% (95% CI: 4.7–7.7%) in 2010 (67 isolates with reduced susceptibility) to 10.3% (95% CI: 8.7–11.9%) in 2014 (146 isolates) (χ2 of the trend: 14.2; p: 0.0002), and the resistance to amikacin was 0.6% (95% CI: 0–1.2%) in 2010 (6 isolates) and from 1.6% (95% CI: 1–2.2%) in 2014 (23 isolates) (χ2 of the trend: 7.2; p=0.007) (Fig. 2).
Resistance to imipenem increased from 0.27% (95% CI: 0–0.6%) in 2010 (3 isolates) to 3.46% (95% CI: 2.5–4.5%) in 2014 (49 isolates) (12.8×; χ2 of the trend: 24.1; p<0.0001). In total, 121 isolates were identified with reduced susceptibility to imipenem, 42 (32.5%) with intermediate susceptibility (MIC=2mg/l) and 79 (67.5%) with high resistance levels (MIC>2mg/l).
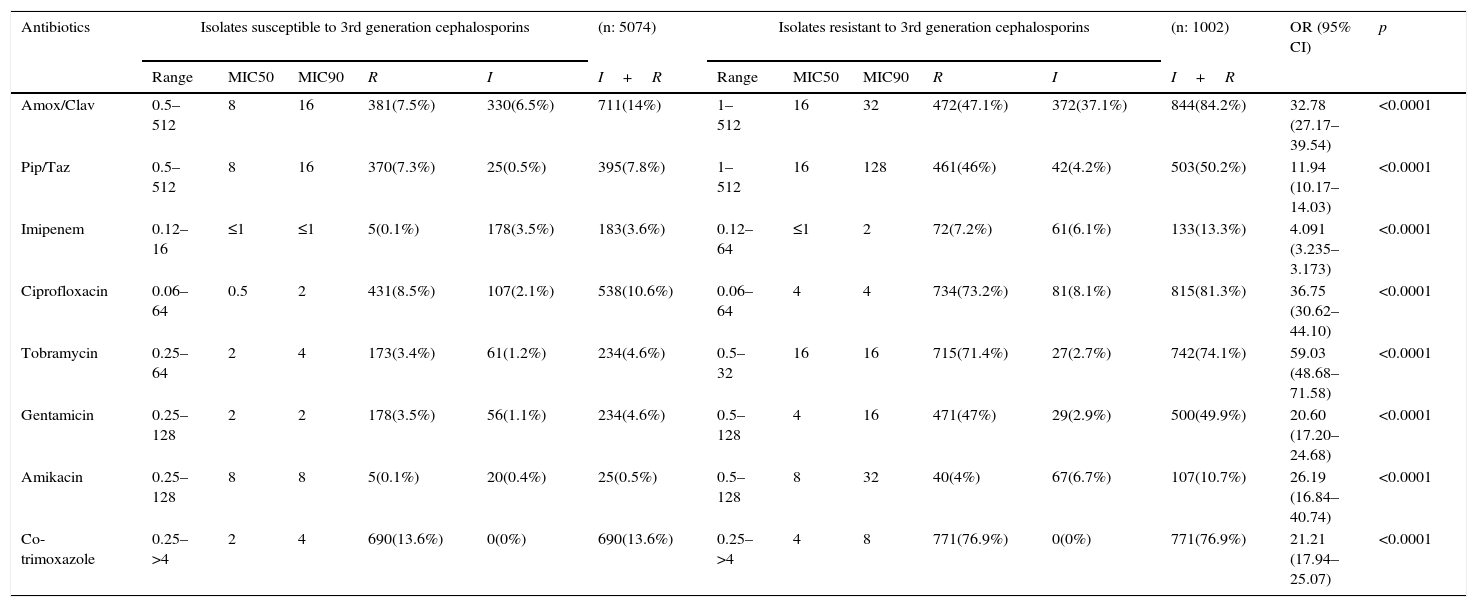
In the 1002 isolates with reduced susceptibility to 3rd generation cephalosporins, a high co-resistance to other antibiotics such as amoxicillin/clavulanic acid, piperacillin/tazobactam, imipenem, ciprofloxacin, tobramycin, gentamicin, amikacin, and co-trimoxazole, was observed. This was significantly higher than the co-resistance which was observed in the isolates sensitive to these antibiotics (Table 1).
Susceptibility to other antibiotics of Klebsiella pneumoniae susceptible and resistant to 3rd generation cephalosporins according to data from the EARS-Net in Spain (2010–2014).
| Antibiotics | Isolates susceptible to 3rd generation cephalosporins | (n: 5074) | Isolates resistant to 3rd generation cephalosporins | (n: 1002) | OR (95% CI) | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | R | I | I+R | Range | MIC50 | MIC90 | R | I | I+R | |||
| Amox/Clav | 0.5–512 | 8 | 16 | 381(7.5%) | 330(6.5%) | 711(14%) | 1–512 | 16 | 32 | 472(47.1%) | 372(37.1%) | 844(84.2%) | 32.78 (27.17–39.54) | <0.0001 |
| Pip/Taz | 0.5–512 | 8 | 16 | 370(7.3%) | 25(0.5%) | 395(7.8%) | 1–512 | 16 | 128 | 461(46%) | 42(4.2%) | 503(50.2%) | 11.94 (10.17–14.03) | <0.0001 |
| Imipenem | 0.12–16 | ≤1 | ≤1 | 5(0.1%) | 178(3.5%) | 183(3.6%) | 0.12–64 | ≤1 | 2 | 72(7.2%) | 61(6.1%) | 133(13.3%) | 4.091 (3.235–3.173) | <0.0001 |
| Ciprofloxacin | 0.06–64 | 0.5 | 2 | 431(8.5%) | 107(2.1%) | 538(10.6%) | 0.06–64 | 4 | 4 | 734(73.2%) | 81(8.1%) | 815(81.3%) | 36.75 (30.62–44.10) | <0.0001 |
| Tobramycin | 0.25–64 | 2 | 4 | 173(3.4%) | 61(1.2%) | 234(4.6%) | 0.5–32 | 16 | 16 | 715(71.4%) | 27(2.7%) | 742(74.1%) | 59.03 (48.68–71.58) | <0.0001 |
| Gentamicin | 0.25–128 | 2 | 2 | 178(3.5%) | 56(1.1%) | 234(4.6%) | 0.5–128 | 4 | 16 | 471(47%) | 29(2.9%) | 500(49.9%) | 20.60 (17.20–24.68) | <0.0001 |
| Amikacin | 0.25–128 | 8 | 8 | 5(0.1%) | 20(0.4%) | 25(0.5%) | 0.5–128 | 8 | 32 | 40(4%) | 67(6.7%) | 107(10.7%) | 26.19 (16.84–40.74) | <0.0001 |
| Co-trimoxazole | 0.25–>4 | 2 | 4 | 690(13.6%) | 0(0%) | 690(13.6%) | 0.25–>4 | 4 | 8 | 771(76.9%) | 0(0%) | 771(76.9%) | 21.21 (17.94–25.07) | <0.0001 |
Table includes isolates collected in the 38 sites that participated during the full study period. The interpretation criteria used were those from the CLSI.
Combined resistance to third-generation cephalosporins, ciprofloxacin and aminoglycosides increased from 3.3% (95% CI: 2.5–4.1%) in 2010 (35 isolates with reduced susceptibility) to 9.7% (95% CI: 7.9–11.5%) in 2014 (137 isolates with reduced susceptibility) (χ2 of the trend: 33.4; p<0.0001) (Fig. 1).
Within the population resistant to 3rd generation cephalosporins, the annual evolution of resistance to other antibiotics was 64.8% in 2010 and 70.9% in 2014 for aminoglycosides (p: 0.7), from 71 to 80% for ciprofloxacin (χ2 of the trend: 5.4; p: 0.01) and from 0.27% in 2010 (3 isolates) to 3.46% in 2014 (49 isolates) for imipenem (12.8×; χ2 of the trend: 20.3; p<0.0001), since 90.7% of the isolates with reduced susceptibility to imipenem also had reduced susceptibility to 3rd generation cephalosporins. The impact of increased resistance to imipenem on the resistance to 3rd cephalosporins, is shown in Fig. 1 (in black in the first group of columns). Although still a minority, said impact is particularly notable from 2012 to 2014 (Fig. 1).
Carbapenemase productionThe 121 isolates detected throughout the study period, with reduced susceptibility to imipenem according to CLSI cut-off points, came from 28 (68.3%) of the 42 participating hospitals. Of these, 104 (86%) were carbapenemase producers: 74 OXA-48, 14 VIM, 9 KPC (6 KPC-2 and 3 KPC-3), 6 IMP and 1 GES. Nevertheless, applying the EUCAST cut-off values for imipenem, only 73 of the 121 isolates (60.3%) would have been categorised as having reduced susceptibility, 40 (54.8%) with intermediate susceptibility (MIC=4–8mg/l) and 33 (45.2%) as resistant (MIC>8mg/l).
The annual evolution of the isolates not susceptible to imipenem according to the CLSI criteria, was as follows: 3 isolates in 2010 (2 VIM-1 and 1 IMP) originating from 3 hospitals; 22 in 2011 (19 OXA-48, 2 VIM-1 and 1 KPC-2) originating from 8 hospitals; 22 in 2012 (11 OXA-48, 3 VIM-1 and 1 KPC-2 and 7 non-susceptible, non-carbapenemase-producing isolates) originating from 9 hospitals; 25 in 2013 (12 OXA-48, 4 VIM, 3 KPC-2, 1 IMP and 1 GES and 4 non-susceptible, non-carbapenemase-producing isolates) originating from 11 hospitals and 49 in 2014 (32 OXA-48, 3 VIM-1, 4 IMP, 3 KPC-3 and 1 KPC-2 and 6 non-susceptible, non-carbapenemase-producing isolates) originating from 18 hospitals.
Outbreaks or endemics [sic] caused by OXA-48-producing K. pneumoniae were detected in 10 different hospitals; with these being responsible for 34 of the isolates, while the remaining 40 were due to sporadic cases. VIM-1-producing isolates were isolated in 9 hospitals, KPC-2-producing ones in 3 hospitals, IMP-producing ones in 3 hospitals and finally, KPC-3- and GES-producing isolates, in one hospital each (Fig. 3).
Isolates with reduced susceptibility to imipenem also had a high prevalence of resistance to cefotaxime (90.7%), followed by ciprofloxacin (82.5%), gentamicin (50.8%), tobramycin (75.7%) and amikacin (12.7%).
DiscussionThe increased resistance to 3rd generation cephalosporins in K. pneumoniae isolates from haemocultures in Europe is a fairly generalised fact in recent years, according to EARS-Net data (http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/pages/index.aspx); in 2014, 17 (58.6%) of the 29 countries that reported information to EARS-Net had resistance levels equal to or exceeding 20%. Nevertheless, the percentages varied widely between Nordic and Central European countries, from low resistance levels, such as Sweden (4.5% in 2014) and the Netherlands (5.5% in 2014), to others with intermediate levels such as the United Kingdom (9.3% in 2014) and Germany (12.7% in 2014). Other countries in Southern Europe have much higher numbers, such as France (30.7% in 2014), Italy (56.5% in 2014) and Greece (72.5% in 2014). As we have seen in Spain, the percentage was 19% in 2014, similar to Belgium which was 19.2%, counting both intermediate and resistant strains (http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2014.pdf, last accessed 11.01.16).
In the 2014 EARS-Net report, 15 of 20 European countries reported on EARS-Net that the production of ESBLs was the main resistance mechanism to 3rd generation cephalosporins in K. pneumoniae with percentages between 85% and 100% of the isolates.5 The increase in ESBL production in K. pneumoniae was mainly due to the CTX-M and SHV families and, to a lesser extent, to the production of plasmid-mediated AmpC β-lactamases, mainly in the CIT and DHA families, both in Europe, including Spain,9,11–15 as well as in other countries.16–18 In France, in a study which compiled data on Escherichia coli and K. pneumoniae strains collected between 2009 and 2013, 20% of the K. pneumoniae isolates were ESBL producers.15 In Canada, the increase was also evident and has gone from 1.5% of K. pneumoniae being ESBL producers in 2007 to 4% in 2011.16
In Korea, between 2005 and 2008 26.2% (294/1121) of K. pneumoniae strains were reported to be resistant to 3rd generation cephalosporins due to ESBL production; the most prevalent type was SHV (73.2%), specifically SHV-12 followed by CTX-M (93.5%), especially CTX-M-14-like and CTX-M-15-like, in this order.17 In Iran, the prevalence of ESBL and AmpC was 28% for both, and the prevalence of the blaCTX-M gene was 13.3% for strains studied between 2007 and 2008.18
In general, according to EARS-Net data, the increase in 3rd generation cephalosporin resistance is related to increases in resistance associated with fluoroquinolones and aminoglycosides. In 2014, 13 (44.8%) of 29 European countries had combined resistance percentages to 3rd generation cephalosporins, fluoroquinolones and aminoglycosides equal to or exceeding 20%. Some of the countries with higher percentages were France (23.8% in 2014), Italy (44.4% in 2014) and Greece (56.8% in 2014). In the case of Spain, as we have seen this percentage was 9.7% in 2014, somewhat higher than Belgium which was 7.9% in 2014.
It is also important to highlight the rapid progression of resistance to 3rd generation cephalosporins in fairly short periods of time, as has occurred for example in France (19.2% in 2010 and 30.7% in 2014) and Italy (47.2% in 2010 and 57.9% in 2014); however, in other countries the resistance percentages are much more stable and even have negative trends, such as the Netherlands (7.2% in 2010 and 5.9% in 2014), Germany (13.4% in 2010 and 13.3% in 2014), Sweden (3.1% in 2010 and 5.8% in 2014) and the United Kingdom (11.2% in 2010 and 10.2% in 2014). (http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2014.pdf, last accessed 11.01.16). As we have seen, in Spain the resistance progression has also been rapid, more than doubling in five years, from 9.4% in 2010 to 19% in 2014.
Co-selection has been reported in resistance mechanisms favoured by joint transmission, both vertically and horizontally, of these mechanisms.19–23 It is a problem with worldwide scope and last November the CDC published guidelines for action for Enterobacteriaceae infections with multiple resistance to the antibiotics used routinely in treatment (http://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf).
Regarding resistance to imipenem, according to EARS-Net, 3 of 28 European countries had resistance percentages higher than 30% in 2014 (Romania 34.5%, Italy 36.2% and Greece 62.7%). However, in most European countries, the resistance percentages are still <5%. Recently, the spreading of carbapenemase-producing K. pneumoniae isolates, including enterobacteriaceae, has been reported in Spain in at least 68% of the provinces, with the possibility of interregional spreading.10 The finding suggests an imminent increase in coming years. In this multicentre study published in 2015, only 9.3% of 379 carbapenemase-producing enterobacteriaceae (74.4% K. pneumoniae) strains were isolated from haemocultures.10 Nevertheless, although the prevalence of isolates from blood is still low in Spain (3.46% in 2014 according to this study), it has progressed rapidly from 0.27% in 2010 – a 12.8-fold increase.
In this paper, we observed that the EUCAST clinical cut-off values for imipenem presented a lower sensitivity for the detection of carbapenemase production than those from the CLSI. However, EUCAST also recommends studying susceptibility to meropenem, data that were not available in this study, as well as application of the epidemiological cut-off values.24
As a possible limitation on the results, the ECDC EARS-Net, with regard to carbapenems, only offered surveillance of imipenem during the study period. In addition, the network uses susceptibility data generated by clinical laboratories based on each laboratory's own methods, usually used for the treatment of patients. EARS-Net also does not collect information about molecular type; however, there is a National Reference Laboratory at the Centro Nacional de Microbiología of the Instituto de Salud Carlos III that does conduct this type of determination making them available to the National Health System.
The increase in resistance to 3rd generation cephalosporins and to carbapenems (mainly due to the production of carbapenemases) constitute two of the most important findings of this study, with significant clinical, epidemiological and public health repercussions.
FundingThe authors have not received any funding to complete this paper.
Conflicts of interestThe authors declare that they have no conflicts of interest.
European Centre for Disease Prevention and Control (ECDC); RD 12/0015, Instituto de Salud Carlos III, Subdirección General de Redes y de Investigación Cooperativa [General Subdirectorate of Networks and Cooperative Research], Ministerio de Economía y Competitividad [Ministry of Economy and Competitiveness]. Collaboration agreement with the Agencia Española del Medicamento y Productos Sanitarios [Spanish Agency of Medicines and Medical Devices] (Reference: MEG 1281/14). Red Española de Investigación en Enfermedades Infecciosas (REIPI [Spanish Network for Research in Infectious Diseases]).
Alejandro González Praetorius (H.G.U. [Hospital General Universitario (General University Hospital)] de Guadalajara), María Teresa Cabezas (H. [Hospital] de Poniente, El Ejido, Almería), Isabel Paz Vidal (Comp. Hopit. [Hospital Complex] de Ourense), Gloria Royo (H.G.U. de Elche, Alicante), Beatriz Orden (H. Universitario [University Hospital] Puerta de Hierro-Majadahonda, Majadahonda, Madrid), Isabel Wilhelmi (H. Severo Ochoa, Leganés, Madrid), Pilar Berdonces (H. Galdakao, Bizkaia), M. Ángeles Mantecón (H. General Yagüe, Burgos), Jesús Viñuelas and Rosario Sánchez-Benito (H. San Pedro de Alcántara, Cáceres), Carmen Amores (H. San Agustín, Linares, Jaén), Francisco José Vasallo (H. Universitario de Vigo, Vigo, Pontevedra), Carmen Raya (H. del Bierzo, Ponferrada, León), María Fe Brezmes (H. Virgen de la Concha, Zamora), Elena Loza (H. Ramón y Cajal, Madrid), M. José González (H. Infantil del Niño Jesús, Madrid), Victoria Pulián and Marta García-Campello (Complejo Hospitalario [Hospital Complex] de Pontevedra), Eugenio Garduño (H. Infanta Cristina, Badajoz), Ángel Campos (H. de Soria), Ana M. Fleites (H.C. [Hospital Clínico (Clinical Hospital)] Asturias, Oviedo), Mar Olga Pérez-Moreno and M. José Centelles Serrano (H. Verge de la Cinta, Tortosa, Tarragona), María Dolores Pérez-Ramírez (H.U. [Hospital Universitario (University Hospital)] Virgen de la Nieves, Granada), Susana Sabater (H.G. de Castellón), Dionisia Fontanals (Corporació Sanitaria Parc Taulí, Barcelona), Natalia Montiel (H. Costa del Sol, Marbella, Málaga), M. Victoria García-López (H. Virgen de la Victoria, Málaga), Juan Carlos Alados and Francisco José Ramírez (H. de Jerez, Cádiz), Carolina Freyre (H. Puerto Real, Cádiz), Ana Isabel Aller (H. Valme, Seville), Inocente Cuesta and Carolina Roldán (H. Ciudad de Jaén), Nieves Gonzalo (H. Vega Baja, Orihuela, Alicante), M. Cruz Villuendas (H. Miguel Servet, Zaragoza), Elena Escribano (Complejo Hospitalario de Albacete), Alberto Delgado-Iribarren (Fundación Hospital Alcorcón [Alcorcón Hospital Foundation], Madrid), Alberto Yagüe (H. La Plana, Villareal, Castellón), Jorge Calvo (H. Marqués de Valdecilla, Santander), Carmen Aspiroz (H. Royo Villanova, Zaragoza), María Antonia Miguel (H.U. Canarias), José Antonio Lepe (H. G. U. Virgen del Rocío, Seville), Antonio Oliver (H. Universitario Son Espases, Palma de Mallorca), Manuel Antonio Rodríguez-Iglesias (H. Universitario Puerta del Mar, Cádiz), Xabier Beristain (Comp. Hosp. de Navarra. Pamplona, Navarra), Isabel Fernández-Natal (Comp. Asist. Universitario [University Care Complex] de León-SACYL [Sanidad de Castilla y León (Castilla y León Healthcare)]).
More information about the components of the Spanish Group of the European Antimicrobial Resistance Surveillance network (EARS-Net) is available in Appendix A.
Please cite this article as: Aracil-García B, Oteo-Iglesias J, Cuevas-Lobato Ó, Lara-Fuella N, Pérez-Grajera I, Fernández-Romero S, et al. Rápido aumento de la resistencia a cefalosporinas de 3a generación, imipenem y de la corresistencia en 7.140 aislados de Klebsiella pneumoniae en hemocultivos (2010-2014) según datos de EARS-Net en España. Enferm Infecc Microbiol Clin. 2017;35:478–484.