Onychomycosis is the main cause of nail alteration. Hepatotoxicity, interference and low adherence to pharmacological treatment are associated. Therefore, our objective was to assess the in vitro effectiveness of tea tree essential oil (less harmful) against main causative agents of these infections.
Material and methodsThrichophyton rubrum and Thrichophyton mentagrophytes were isolated and inoculated at a concentration of 3 × 105 CFU/mL in potato agar dextrose and tea tree essential oil at different concentrations to assess its effect by counting colony forming units and radial growth.
ResultsTrichophyton rubrum growth inhibition was obtained at concentrations higher than 0.04% of the essential tea tree oil (p = 0.004). In the case of Thrichophyton mentagrophytes, inhibition was obtained at 0.02% (p = 0.017), and even complete inhibition at a final concentration of the oil at 0.07%.
ConclusionsTea tree essential oil inhibits the in vitro growth of the fungus and may be a less harmful alternative to the onychomycosis treatment.
La onicomicosis por dermatofitos son la principal causa de alteración ungueal. Su abordaje terapéutico farmacológico tiene asociado bajas tasas de éxito, hepatotoxicidad, interferencia y baja adherencia al tratamiento. Por ello, nuestro objetivo fue valorar la efectividad in vitro del aceite esencial de árbol de té, para aportar alternativas menos nocivas en el abordaje terapéutico frente a los principales agentes causantes de estas infecciones.
Material y métodosSe aisló de fragmentos de uña con infección fúngica el Thrichophyton rubrum y Thrichophyton mentagrophytes. Posteriormente, se inocularón a una concentración de 3 × 10⁵ UFC/mL en agar patata con dextrosa y aceite esencial de árbol de té a diferentes concentraciones para evaluar su efecto mediante el recuento de unidades formadoras de colonia (UFC) y el crecimiento radial (CR).
ResultadosSe obtuvo compromiso en el crecimiento deTrichophyton rubrum a concentraciones a partir de 0.04% del aceite esencial de árbol de té (p = 0,004). En el caso del Thrichophyton mentagrophytes se obtuvo inhibición a partir de 0.02% (p = 0,017), e incluso la inhibición completa a una concentración final del aceite a 0,07%.
ConclusionesEl aceite esencial de árbol de té inhibe el crecimiento in vitro de los hongos estudiados, pudiéndose considerar una alternativa menos nociva para el abordaje terapéutico de las onicomicosis.
Dermatophytes, particularly Trichophyton rubrum and Trichophyton mentagrophytes, are the main causative agents of severe onychomycosis. Onychomycosis has an estimated incidence of 2%–8% in the global population, with the infection predominantly affecting the big toenail. That incidence range depends on the study population, lifestyles and current high levels of states of immunosuppression (individuals with human immunodeficiency virus [HIV] infection or diabetes, transplant recipients and individuals on corticosteroids)1,2.
Onychomycosis is responsible for around 50% of all nail abnormalities and is associated with significant negative psychological effects as nails are considered to be a symbol of beauty. This causes people with onychomycosis to suffer from anxiety, self-rejection and social isolation and to demand fast, effective treatment3,4.
The treatments of choice for superficial fungal infections are systemic and topical drugs, which account for a global expenditure of around five million United States dollars5,6. They are long-term treatments (6–12 months) and a multitude of potentially hepatotoxic drug interactions have been reported. Moreover, the cure rate of these therapies is questionable and they are associated with high withdrawal rates due to their pharmacokinetics and poor patient compliance, resulting in unsuccessful treatment6,7.
To minimise these drawbacks, the effects of many natural extracts in reducing the toxicity of these drugs are being studied8–10. One such natural extract is tea tree essential oil, which exerts an antibacterial effect against a large number of bacteria, an antifungal effect against Candida and an antiviral effect against the herpes simplex virus11.
Despite the potential use of many essential oils to treat skin infections11, studies must be conducted on tea tree essential oil (Melaleuca alternifolia) to evaluate its efficacy and effectiveness against onychomycosis. In this light, in order to provide less harmful alternatives, this study examines the effect in vitro of tea tree essential oil versus Trichophyton rubrum and Trichophyton mentagrophytes, the main causative agents of nail infection1,2.
Material and methodsSpecies isolationThe fungi were isolated from a nail infected by Trichophyton rubrum and another nail infected by Trichophyton mentagrophytes. The nails in question were cultivated in Sabouraud chloramphenicol agar and then in potato dextrose agar (PDA). The DNA of the fungus was extracted and amplified by PCR12. Finally, genetic confirmation was performed by sequencing.
Solubilisation of the oil and incorporation into the growth mediumNaissance tea tree essential oil was used. In order to incorporate it into PDA, it was initially solubilised with PanReac AppliChem dimethyl sulfoxide (DMSO) at 10%. To attain a stable emulsion and promote the incorporation of the oil into the growth medium, DMSO was added until a final concentration of 0.112% was achieved in the Petri dish. For this same purpose, tests were conducted by adding various concentrations of Tween® 20 (Polysorbate, from Sigma-Aldrich®), although its use was ultimately abandoned as it altered the morphology of Trichophyton rubrum.
Preparation of the inoculumThe spores were extracted and the inoculum prepared as described by EUCAST13.
Sensitivity studyFirst, it was necessary to rule out an inhibitory or toxic effect of DMSO on fungal growth at the concentration at which a stable emulsion of oil in the growth medium was achieved. To do so, 10 μl of the prepared inoculum of each strain was seeded all together at a concentration of 3 × 105 CFU/mL for the colony-forming unit (CFU) study. 5 μl of the same inoculum was also inoculated to evaluate radial growth (RG) in PDA and in PDA with DMSO. They were incubated at 30 °C for six days before conducting the CFU count and measuring RG.
Once it had been established that the concentration margins of DMSO did not affect fungal growth, the fungus was inoculated in PDA with a stable emulsion of tea tree oil at different concentrations. These tests were performed in duplicate and repeated four times.
Statistical studyThe results were analysed with SPSS ver. 21.0, applying Student’s t test for independent samples and establishing the level of statistical significance at p < 0.05.
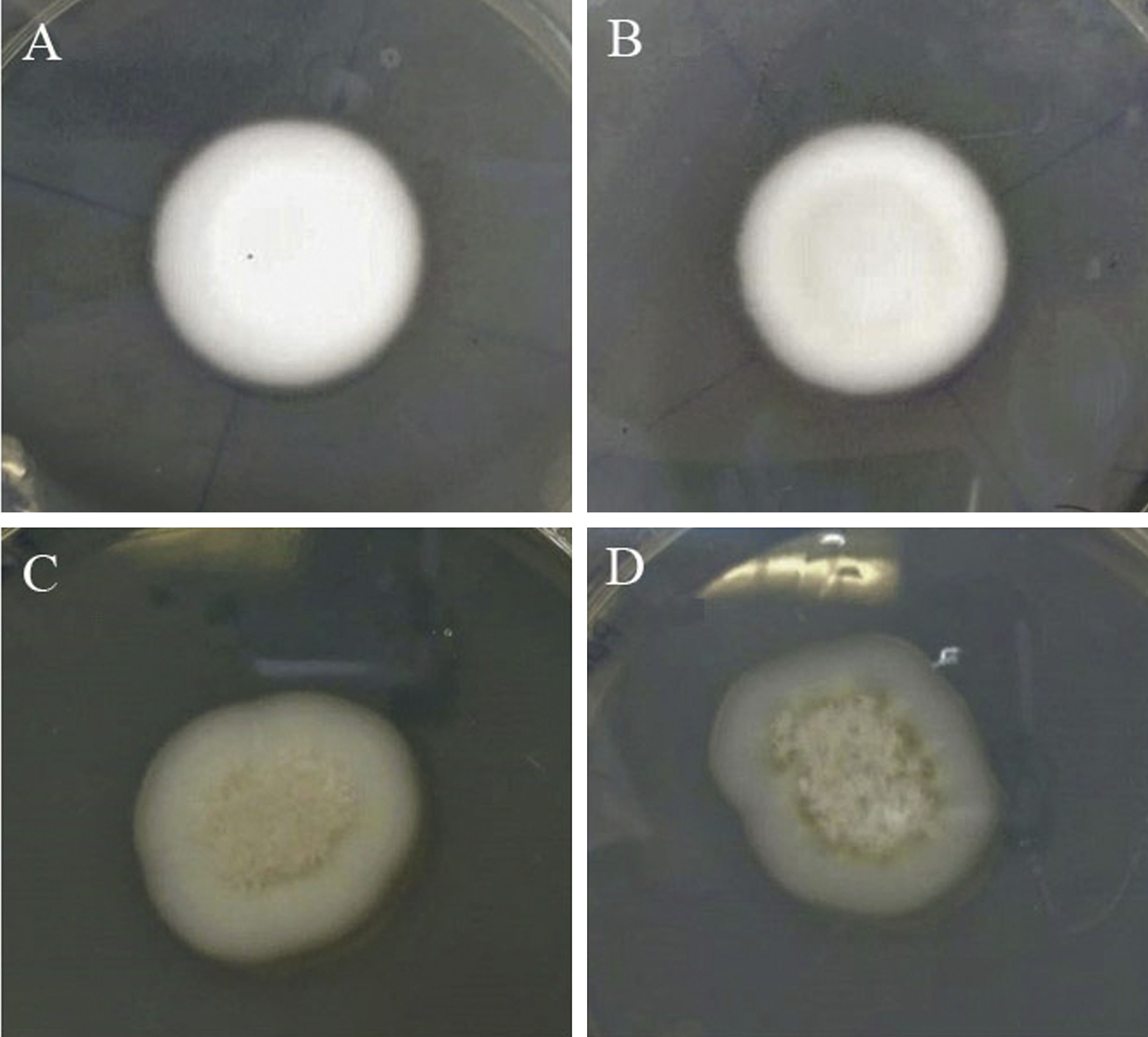
ResultsThe oil was solubilised with DMSO and Tween for incorporation into the solid medium according to the methods described in the articles consulted11,14,15. The results showed that, with Tween 20, the Trichophyton rubrum isolated underwent morphological changes, possibly due to its toxicity (Fig. 1). A stable emulsion of tea tree oil in the medium was finally attained at a final DMSO concentration of 0.112%. No statistically significant differences were found when the results obtained in the medium with and without solvent were compared (Table 1), leading to the conclusion that DMSO at that concentration did not affect the growth of the fungi studied.
Growth morphology of the Trichophyton rubrum colony isolated under different conditions. (A) Normal growth is observed in PDA. (B) No alteration is observed when PDA contains DMSO. (C) Alterations are observed when PDA contains DMSO and Tween 20 at 0.1%. (D) Alterations are also observed in PDA alone with Tween 20 at 0.1%.
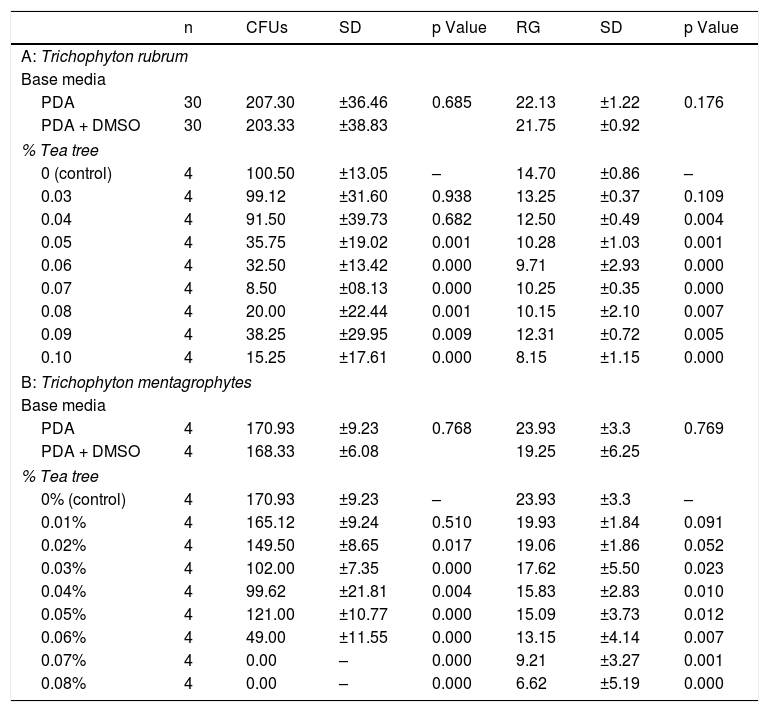
Descriptive statistical study and comparison of growth media with Student's t test for independent samples, CFUs and RG under the different study conditions. (A) Results obtained by comparing the base medium and adding the solvent, DMSO at a final concentration of 0.112%. (B) Results obtained at the different tea tree oil concentrations with a constant final DMSO concentration of 0.112%.
| n | CFUs | SD | p Value | RG | SD | p Value | |
|---|---|---|---|---|---|---|---|
| A: Trichophyton rubrum | |||||||
| Base media | |||||||
| PDA | 30 | 207.30 | ±36.46 | 0.685 | 22.13 | ±1.22 | 0.176 |
| PDA + DMSO | 30 | 203.33 | ±38.83 | 21.75 | ±0.92 | ||
| % Tea tree | |||||||
| 0 (control) | 4 | 100.50 | ±13.05 | – | 14.70 | ±0.86 | – |
| 0.03 | 4 | 99.12 | ±31.60 | 0.938 | 13.25 | ±0.37 | 0.109 |
| 0.04 | 4 | 91.50 | ±39.73 | 0.682 | 12.50 | ±0.49 | 0.004 |
| 0.05 | 4 | 35.75 | ±19.02 | 0.001 | 10.28 | ±1.03 | 0.001 |
| 0.06 | 4 | 32.50 | ±13.42 | 0.000 | 9.71 | ±2.93 | 0.000 |
| 0.07 | 4 | 8.50 | ±08.13 | 0.000 | 10.25 | ±0.35 | 0.000 |
| 0.08 | 4 | 20.00 | ±22.44 | 0.001 | 10.15 | ±2.10 | 0.007 |
| 0.09 | 4 | 38.25 | ±29.95 | 0.009 | 12.31 | ±0.72 | 0.005 |
| 0.10 | 4 | 15.25 | ±17.61 | 0.000 | 8.15 | ±1.15 | 0.000 |
| B: Trichophyton mentagrophytes | |||||||
| Base media | |||||||
| PDA | 4 | 170.93 | ±9.23 | 0.768 | 23.93 | ±3.3 | 0.769 |
| PDA + DMSO | 4 | 168.33 | ±6.08 | 19.25 | ±6.25 | ||
| % Tea tree | |||||||
| 0% (control) | 4 | 170.93 | ±9.23 | – | 23.93 | ±3.3 | – |
| 0.01% | 4 | 165.12 | ±9.24 | 0.510 | 19.93 | ±1.84 | 0.091 |
| 0.02% | 4 | 149.50 | ±8.65 | 0.017 | 19.06 | ±1.86 | 0.052 |
| 0.03% | 4 | 102.00 | ±7.35 | 0.000 | 17.62 | ±5.50 | 0.023 |
| 0.04% | 4 | 99.62 | ±21.81 | 0.004 | 15.83 | ±2.83 | 0.010 |
| 0.05% | 4 | 121.00 | ±10.77 | 0.000 | 15.09 | ±3.73 | 0.012 |
| 0.06% | 4 | 49.00 | ±11.55 | 0.000 | 13.15 | ±4.14 | 0.007 |
| 0.07% | 4 | 0.00 | – | 0.000 | 9.21 | ±3.27 | 0.001 |
| 0.08% | 4 | 0.00 | – | 0.000 | 6.62 | ±5.19 | 0.000 |
*CFUs: colony-forming units. SD: standard deviation. RG: radial growth in mm.
The CFU count and RG measurement in the media with the different concentrations of tea tree essential oil were then taken. A comparison of the results obtained in the control media and the media with oil revealed a statistically significant difference in the CFU count at oil concentrations greater than or equal to 0.05% (p = 0.001), and in RG at concentrations greater than or equal to 0.04% (p = 0.004), for Trichophyton rubrum (Table 1A). In contrast, according to the CFU count, Trichophyton mentagrophytes growth was compromised at oil concentrations in excess of 0.02% (p = 0.017) and inhibited at concentrations greater than 0.07% (Table 1B). With regard to the RG of Trichophyton mentagrophytes, evidence of growth at concentrations greater than or equal to 0.03% (p = 0.023) was found.
DiscussionThe confined conditions to which feet are exposed are predisposing factors for onychomycosis1,2. Hence, this sensitivity study was conducted on a fungus isolated from a human nail sample in order to obtain strains adapted to these conditions and reflect the real world as closely as possible.
The first results obtained showed that the Tween in the growth medium caused the Trichophyton rubrum isolated to undergo morphological changes, suggesting that the antifungal effect reported in prior studies may have been due not to the use of the oil alone, but potentially to its combination with Tween11,14,15. This study provides conclusive and objective data to confirm the fungistatic effect in vitro of tea tree oil against the main causative agents of onychomycosis. The tea tree oil was finally solubilised with DMSO alone at a final concentration of 0.112%. The lack of statistical significance when comparing the growth results obtained in both the absence and presence of DMSO showed that DMSO does not inhibit the growth of Trichophyton rubrum or Trichophyton mentagrophytes. This finding can be considered to be consistent with that reported in the literature reviewed11,14,15. However, these studies reported a higher final DMSO concentration, between 8% and 20%, and in some studies Tween was also added to encourage the incorporation of the oil into the medium15. This study obtained effective and objective results attesting to the antifungal effect in less toxic media than prior studies that favoured cell viability, with no morphological changes being observed11,14,15.
Finally, although application of essential oils is a popular treatment for fungal infections of the skin and its appendages around the world11, studies such as this one provide the foundation for developing treatments that minimise the interference and toxic effects of commonly used antifungals, particularly azoles2. Further studies are necessary to quantify the exact inhibitory concentration of tea tree oil against Trichophyton rubrum, Trichophyton mentagrophytes and other common subspecies; studies that examine the permeability of nails to these oils are also needed. Finally, clinical trials should also be conducted in order to obtain effective treatments with no or negligible risk of toxicity for patients with onychomycosis.
ConclusionTea tree essential oil exerts a fungistatic effect in vitro against the dermatophyte fungus Trichophyton rubrum at a concentration greater than or equal to 0.04%. Against Trichophyton mentagrophytes, it exerts an antifungal action at a concentration of 0.02% and demonstrates a potentially inhibitory function at a concentration of 0.07%.
FundingThis study was funded by the Regional Government of Extremadura (Spain) and the European Regional Development Fund (ERDF) through a grant to the research group (code CTS020, reference GR18182), as well as by assistance to promote R&D&I through the mobility of post-doctoral researchers in 2017 (File P017018). To conduct this study, Pablo González García received a grant (contract reference 420/2018) for Research Support Staff funded by Servicio Extremeño Público de Empleo [Extremadura Public Employment Service] (SEXPE) and the Regional Government of Extremadura.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marcos-Tejedor F, González-García P, Mayordomo R. Solubilización in vitro del aceite de árbol de té y primeros resultados de su efecto antifúngico en onicomicosis. Enferm Infecc Microbiol Clin. 2021;39:395–398.