The development of resistances to numerous antimicrobial agents by different pathogens is a serious problem in public health, as they limit the treatment options in infections caused by these pathogens to a remarkable extent. Moreover, the scant contribution of new molecules capable of acting against these modified strains has resulted in the need to explore other pathways, such as the recovery of old antimicrobial agents and associations of others which may turn out to be active.1,2 Along these lines, fosfomycin is an antibacterial agent which has been known for a number of decades; it is active against a wide range of microorganisms, including strains of multi-resistant gram-negative bacilli. Nonetheless, the principal limitation on the use of fosfomycin in the treatment of systemic infections is the development of resistance during therapy, associated with the high frequency of mutations observed in different in vitro studies,3 although this limitation may be prevented by using the antibiotic in combination with other molecules, obtaining, at the same time, synergic antibacterial activity.2,4 Although the data obtained in vitro are suggestive and seem to point in this direction, its translation into clinical practice is not very common. In this study we present a case of surgical mesh infection by a strain of carbapenemase-producing Enterobacter cloacae, refractory to different antimicrobial regimens, which was finally treated successfully with a combination of ciprofloxacin and fosfomycin that presented synergic activity to the microorganism that had previously been demonstrated in the laboratory.
The subject was a 75-year-old, hypertensive, asthmatic woman who had recently been admitted owing to flare-ups of her COPD. During one such flare-up, she developed intestinal incarceration from a prior supra-umbilical hernia. A surgical intervention was performed, with the placement of a mesh, which in the immediate post-operative period showed signs of infection with the formation of a fistula. A number of different microorganisms were isolated in the culture from the exudate of the fistula; these were treated with different antimicrobial regimens, but with no sustained clinical improvement. In the final exudate sample taken, a strain of VIM-type carbapenemase-producing Enterobacter cloacae was isolated, initially detected by phenotypic methods and subsequently confirmed by PCR, through the amplification of an internal fragment of the blaVIM gene (350nt); the amplicon obtained was not sequenced, owing to which the specific enzymatic variant was not defined. The strain was only sensitive to tigecycline and aminoglycosides. An abdominal CT scan showed a fistula between the transverse colon and a mass of soft tissue adjacent to the surgical mesh, with antimicrobial treatment being started with amikacin (750mg/day) and parenteral tigecycline (starting dose 100mg, followed by 50mg every 12h). Two months later, the patient underwent further surgery, partially extracting the infected mesh and closing the fistula. The patient developed an episode of reversible acute renal failure and on post-operative day nine presented a fever spike, with VIM-producing Enterobacter cloacae once again being isolated in the exudate from the fistula, now resistant to tigecycline and only sensitive to amikacin. In light of the persistence of the infra-umbilical tissue mass, once again observed through a CT scan, a combination of parenteral ciprofloxacin (400mg/8h) and parenteral fosfomycin (1g/8h) was prescribed, since the association had been shown to be synergic for the microorganism, through both combined diffusion gradients (E-Test) and through the design of lethality curves with the aforesaid antimicrobial agents. At 24h, the patient remained afebrile, with a clinical and analytical improvement being observed over the following days. One month later, upon discharge, the treatment was replaced with ciprofloxacin 500mg/12h and fosfomycin 500mg/8h, both taken orally. Treatment was suspended after one year, with the patient remaining asymptomatic since then.
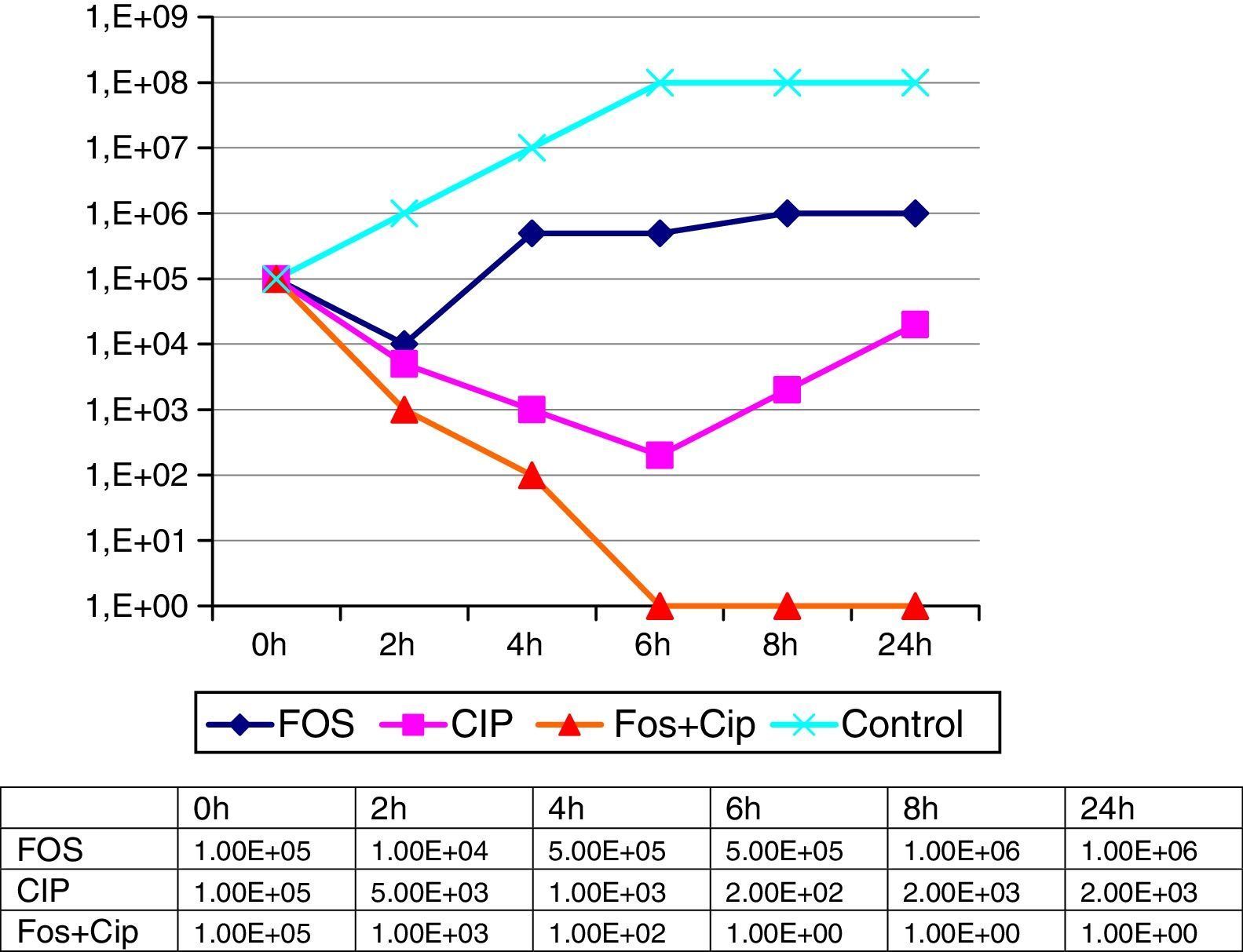
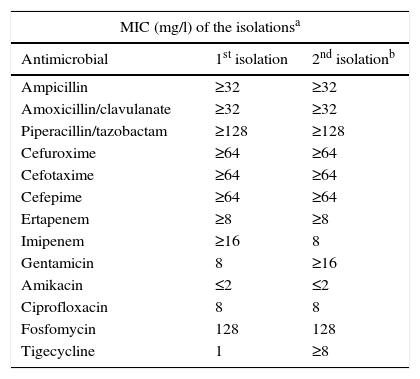
The minimal inhibitory concentrations of the isolated strains were the same on both occasions, presenting a pattern of high resistance to the majority of the antimicrobial agents tested (Table 1). The phenotypic analyses of the isolations presented identical patterns, suggestive in both cases of a metalocarbapenemase-producing strain. The activity of fosfomycin and ciprofloxacin, both alone and in combination, was ascertained by means of a dynamic study of the lethality curves using an initial inoculum of 5×105cfu/ml of the microorganism on the basis of the corresponding MICs of both compounds. The number of CFUs was determined at 2, 4, 6, 8 and 24h, with a synergic affect being considered as a 100-times reduction in the number of cfu/ml with the combination compared to the most active individual component of the combination (Fig. 1). In these lethality curves, an initial reduction in bacterial growth was observed when individually testing the 2 components of the combination, although as of 2h for fosfomycin and 6h for ciprofloxacin, an overgrowth similar to or greater than the initial inoculum was observed. The synergic affect of the two drugs in combination, on the other hand, was evinced as of 2h, increasing progressively, with no regrowth being observed in any of the time stages established up to 24h. Similarly, the possibility of synergy for the microorganism with fosfomycin+amikacin, which is a combination studied in the literature, was also studied in this case, finding amikacin to offer a low MIC when administered individually. Nonetheless, the combination only presented a slight additive effect, owing to which it was ruled out when implementing the combined treatment.
Sensitivity patterns for a strain of VIM-producing Enterobacter cloacae in the infection of a surgical mesh.
| MIC (mg/l) of the isolationsa | ||
|---|---|---|
| Antimicrobial | 1st isolation | 2nd isolationb |
| Ampicillin | ≥32 | ≥32 |
| Amoxicillin/clavulanate | ≥32 | ≥32 |
| Piperacillin/tazobactam | ≥128 | ≥128 |
| Cefuroxime | ≥64 | ≥64 |
| Cefotaxime | ≥64 | ≥64 |
| Cefepime | ≥64 | ≥64 |
| Ertapenem | ≥8 | ≥8 |
| Imipenem | ≥16 | 8 |
| Gentamicin | 8 | ≥16 |
| Amikacin | ≤2 | ≤2 |
| Ciprofloxacin | 8 | 8 |
| Fosfomycin | 128 | 128 |
| Tigecycline | 1 | ≥8 |
In recent years, the combination of fosfomycin with other antimicrobial agents has frequently been cited.5–7 Among the associations tested, the combination with ciprofloxacin has offered acceptable results, with the synergic activity between both antimicrobial agents being correlated with factors such as the probable postantibiotic effect for ciprofloxacin8 and the reduction of the bacterial inoculum by the action of fosfomycin, allowing the fluoroquinolone to express its activity against a reduced number of active microorganisms.9,10 In our case, the monotherapy initially established served, credibly, to select the strain of multi-resistant Enterobacter cloacae, which did not respond to the treatment subsequently established with amikacin and tigecycline, and had to be suspended owing to the onset of renal failure, on top of the development of resistance to tigecycline. Nonetheless, once the determinations of the fosfomycin–ciprofloxacin combination had been performed, and the latter treatment initiated, on the following day the patient remained afebrile, with a clear clinical and analytical improvement being progressively observed along with the disappearance of the microorganism from the subsequent cultures. The treatment was maintained for one year, owing to the impossibility of totally removing the mesh. After this period, the patient remained asymptomatic, the infra-umbilical tissue mass disappeared and the microorganism did not recover in subsequent controls.
This therapeutic regime is probably not extendible to all patients with deep infections owing to carbapenemase-producing, or simply multi-resistant, microorganisms, since the synergy encountered is not repeated in in vitro series in all cases.4,7 Nonetheless, it does highlight the need, wherever possible, to “personalise” combined antimicrobial therapy, as long as there are no alternatives with sufficiently validated monotherapies for the treatment of infections caused by these microorganisms with high and extended levels of resistance.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To the Antibiotic Resistance Monitoring Programme of the National Centre for Microbiology for the molecular characterisation of the carbapenemase.
Please cite this article as: Gómez-Garcés JL, Gil-Romero Y, Vazquez O, Merino F. Actividad sinérgica y eficacia clínica de la asociación fosfomicina-ciprofloxacino en el tratamiento de una infección de malla quirúrgica con absceso de partes blandas por Enterobacter cloacae productor de carbapenemasa. Enferm Infecc Microbiol Clin. 2017;35:135–136.