The aim of this study was to assess the prognostic value of vitamin D, vitamin D binding protein (VDBP) and vitamin D-related peptides in septic shock patients in relation to hospital mortality.
MethodsThis is a single-center, prospective, observational study that included all consecutive patients meeting criteria for septic shock who were admitted to the ICU. VDBP, 25-hydroxy vitamin D, 1,25-dihydroxy vitamin D, cathelicidin and beta-defensin levels were determined in blood samples obtained on admission to the ICU.
ResultsSeventy-five patients were studied. The best area under the curve (AUC) for prediction of in-hospital mortality was for VDBP (0.78), with a negative predictive value of 85.45% for the optimal cut-off point. VDBP was also the only variable that had a statistically significant association with a higher risk of in-hospital mortality, regardless of other assessed variables and pre-determined confounders: adjusted odds ratio of 5.20 (95% confidence interval: 1.21–22.36). When restricted to patients with vitamin D insufficiency (n=54), the AUC was 0.77, and the adjusted OR 12.22 (95% CI: 1.46–102.14; p=0.021) for in-hospital mortality.
ConclusionsVDBP levels showed a statistically significant association with in-hospital mortality, supporting the clinical utility of VDBP as a good prognostic marker in septic shock patients. Vitamin D and vitamin D-related peptides are not associated with in-hospital mortality. These results should be confirmed in a multicentre study with a larger sample size.
El objeto de este estudio fue evaluar el valor pronóstico de la vitamina D, la proteína transportadora de la vitamina D (PTVD) y de los péptidos derivados de la vitamina D en relación a la mortalidad hospitalaria de los pacientes en shock séptico.
MétodosSe trata de un estudio prospectivo unicéntrico observacional que incluyo consecutivamente a todos los pacientes que ingresan en la UCI en shock séptico. Los valores de la PTVD, 25-hydroxy vitamina D, 1,25-dihydroxy vitamina D, catelicidina y beta-defensina se cuantificaron en muestras sanguíneas extraídas en el momento del ingreso en la UCI.
ResultadosSe incluyeron un total de 75 pacientes. La mejor área bajo la curva (AUC) para la predicción de mortalidad hospitalaria fue para la PTVD (0,78), con un valor predictivo negativo del 85,45% para el punto de corte óptimo. La PTVD fue además la única variable asociada estadísticamente con un mayor riesgo de mortalidad hospitalaria independientemente de las otras variables evaluadas y de los factores de confusión prestablecidos: odds ratio ajustada 5,20 (intervalo de confianza [IC] 95%: 1,21-22,36). Cuando el estudio se focalizó en los pacientes con insuficiencia de vitamina D (N=54), el AUC fue de 0,77 y la odds ratio ajustada de 12,22 (IC 95%: 1,46-102,14; p=0,021) en relación a la mortalidad hospitalaria.
ConclusionesLos valores sanguíneos de la PTVD se asociaron de forma estadísticamente significativa con la mortalidad hospitalaria, apoyando la potencial utilidad clínica de la PTVD como un buen marcador pronóstico en los pacientes en shock séptico. La vitamina D y los péptidos derivados de ella no relacionaron con la mortalidad hospitalaria. Estos resultados deben ser confirmados en un estudio multicéntrico con mayor número de pacientes.
There is increasing evidence regarding the pivotal role played by vitamin D in health and disease. Although the primary and most well-known function of vitamin D is to maintain calcium and phosphorus homeostasis, and promote bone mineralization, there is a body of literature focusing on the pleiotropic effects of the vitamin, such as immune modulation, endothelial and mucosal functions, and glucose metabolism. Thus, vitamin D deficiency has been associated with myocardial infarction, diabetes, autoimmune disease, chronic obstructive pulmonary disease, neoplasm, tuberculosis, and increased mortality in the general population.1
Recent studies in critically-ill patients have suggested that vitamin D deficiency may be associated with sepsis development and poorer outcomes.2–5 However, the exact mechanism explaining this association is still unclear. Vitamin D has autoimmune activity, but also acts as an intermediary in the generation of antimicrobial peptides (cathelicidins and defensins) by monocytes/macrophages.6 Moreover, some other components of the vitamin D axis, such as vitamin D binding protein (VDBP), have anti-inflammatory and immunomodulatory functions that may affect these outcomes.7
The aim of this study was to assess the prognostic value of vitamin D, VDBP and vitamin D-related peptides in septic shock patients in relation to hospital mortality.
Materials and methodsStudy design and settingWe conducted a single-center prospective observational study in a 30-bed adult intensive care unit (ICU) at Marques de Valdecilla University Hospital in Spain, between April 2015 and April 2016. Eligible patients were all consecutive adult patients, aged 18 or older and admitted to ICU with septic shock according to the Third International Consensus Definitions.8 We excluded patients under 18 years of age, as well as individuals with recent cardiac arrest or with withdraw of life sustaining decision and patients without septic shock at the time of admission to the ICU but who developed such problem during admission. Clinical and demographic characteristics of all patients, including age, gender, immunosuppression (AIDS, neutropenia [neutrophil count<1×109/L], exposure to glucocorticoids [>0.5mg/kg for >30d] and/or immunosuppressive or cytotoxic medications, solid organ transplantation, allogeneic or autologous stem cell transplantation, hematological malignancy, or solid tumor), Acute Physiology and Chronic Health Evaluation II score at 24h and Sequential Organ Failure Assessment score at admission were recorded (Table 1). The local ethics committee (CEIC Cantabria 2014.159) approved this study, and informed consent was obtained for all patients.
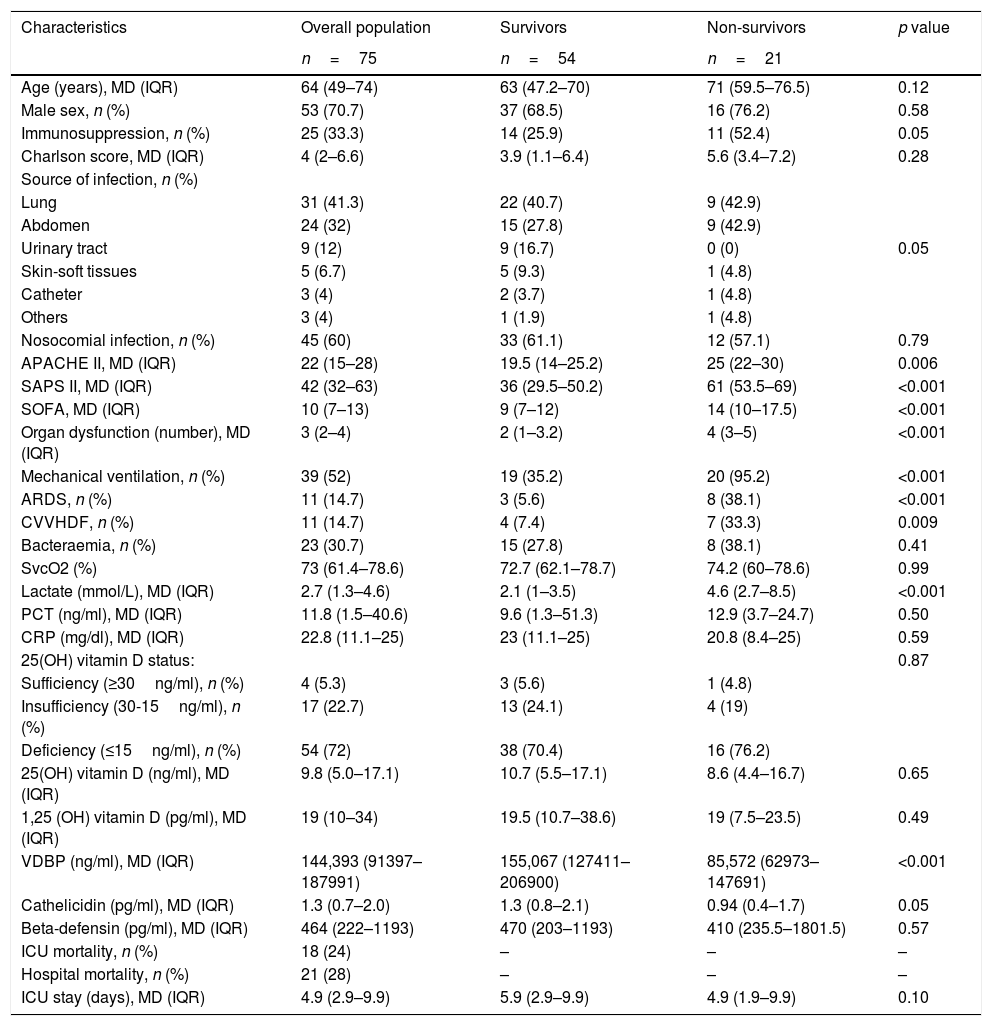
Baseline characteristics of the study population.
| Characteristics | Overall population | Survivors | Non-survivors | p value |
|---|---|---|---|---|
| n=75 | n=54 | n=21 | ||
| Age (years), MD (IQR) | 64 (49–74) | 63 (47.2–70) | 71 (59.5–76.5) | 0.12 |
| Male sex, n (%) | 53 (70.7) | 37 (68.5) | 16 (76.2) | 0.58 |
| Immunosuppression, n (%) | 25 (33.3) | 14 (25.9) | 11 (52.4) | 0.05 |
| Charlson score, MD (IQR) | 4 (2–6.6) | 3.9 (1.1–6.4) | 5.6 (3.4–7.2) | 0.28 |
| Source of infection, n (%) | 0.05 | |||
| Lung | 31 (41.3) | 22 (40.7) | 9 (42.9) | |
| Abdomen | 24 (32) | 15 (27.8) | 9 (42.9) | |
| Urinary tract | 9 (12) | 9 (16.7) | 0 (0) | |
| Skin-soft tissues | 5 (6.7) | 5 (9.3) | 1 (4.8) | |
| Catheter | 3 (4) | 2 (3.7) | 1 (4.8) | |
| Others | 3 (4) | 1 (1.9) | 1 (4.8) | |
| Nosocomial infection, n (%) | 45 (60) | 33 (61.1) | 12 (57.1) | 0.79 |
| APACHE II, MD (IQR) | 22 (15–28) | 19.5 (14–25.2) | 25 (22–30) | 0.006 |
| SAPS II, MD (IQR) | 42 (32–63) | 36 (29.5–50.2) | 61 (53.5–69) | <0.001 |
| SOFA, MD (IQR) | 10 (7–13) | 9 (7–12) | 14 (10–17.5) | <0.001 |
| Organ dysfunction (number), MD (IQR) | 3 (2–4) | 2 (1–3.2) | 4 (3–5) | <0.001 |
| Mechanical ventilation, n (%) | 39 (52) | 19 (35.2) | 20 (95.2) | <0.001 |
| ARDS, n (%) | 11 (14.7) | 3 (5.6) | 8 (38.1) | <0.001 |
| CVVHDF, n (%) | 11 (14.7) | 4 (7.4) | 7 (33.3) | 0.009 |
| Bacteraemia, n (%) | 23 (30.7) | 15 (27.8) | 8 (38.1) | 0.41 |
| SvcO2 (%) | 73 (61.4–78.6) | 72.7 (62.1–78.7) | 74.2 (60–78.6) | 0.99 |
| Lactate (mmol/L), MD (IQR) | 2.7 (1.3–4.6) | 2.1 (1–3.5) | 4.6 (2.7–8.5) | <0.001 |
| PCT (ng/ml), MD (IQR) | 11.8 (1.5–40.6) | 9.6 (1.3–51.3) | 12.9 (3.7–24.7) | 0.50 |
| CRP (mg/dl), MD (IQR) | 22.8 (11.1–25) | 23 (11.1–25) | 20.8 (8.4–25) | 0.59 |
| 25(OH) vitamin D status: | 0.87 | |||
| Sufficiency (≥30ng/ml), n (%) | 4 (5.3) | 3 (5.6) | 1 (4.8) | |
| Insufficiency (30-15ng/ml), n (%) | 17 (22.7) | 13 (24.1) | 4 (19) | |
| Deficiency (≤15ng/ml), n (%) | 54 (72) | 38 (70.4) | 16 (76.2) | |
| 25(OH) vitamin D (ng/ml), MD (IQR) | 9.8 (5.0–17.1) | 10.7 (5.5–17.1) | 8.6 (4.4–16.7) | 0.65 |
| 1,25 (OH) vitamin D (pg/ml), MD (IQR) | 19 (10–34) | 19.5 (10.7–38.6) | 19 (7.5–23.5) | 0.49 |
| VDBP (ng/ml), MD (IQR) | 144,393 (91397–187991) | 155,067 (127411–206900) | 85,572 (62973–147691) | <0.001 |
| Cathelicidin (pg/ml), MD (IQR) | 1.3 (0.7–2.0) | 1.3 (0.8–2.1) | 0.94 (0.4–1.7) | 0.05 |
| Beta-defensin (pg/ml), MD (IQR) | 464 (222–1193) | 470 (203–1193) | 410 (235.5–1801.5) | 0.57 |
| ICU mortality, n (%) | 18 (24) | – | – | – |
| Hospital mortality, n (%) | 21 (28) | – | – | – |
| ICU stay (days), MD (IQR) | 4.9 (2.9–9.9) | 5.9 (2.9–9.9) | 4.9 (1.9–9.9) | 0.10 |
Apache II: Acute Physiology and Chronic Health disease Classification System II; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; ARDS: acute respiratory distress syndrome; CVVHDF: continuous venovenous hemodiafiltration; SvcO2: central venous oxygen saturation; PCT: procalcitonin; CRP: C reactive protein; VDBP: vitamin D binding protein. MD (IQR); median (interquartile range). p results based on Mann–Whitney U test.
A single blood sample was performed coinciding with the first clinical extraction for clinical purposes at the moment of admittance of the patient in the ICU. Serum and plasma samples were collected in the appropriate way for the different assays and aliquots for this specific study were stored at −80°C until assayed. 25-hydroxy vitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH2D)) levels were measured by automated competitive chemiluminiscence assay (Liaison XL, DiaSorin Inc, Stillwater MN USA). Our laboratory is DEQAS (Vitamin D External Quality Assessment Scheme) certified for both parameters. Vitamin D deficiency and insufficiency were defined as serum 25(OH)D levels less than 15ng/mL and 30ng/ml respectively. 1,25-dihydroxyvitamin D (1,25(OH2D)) levels were measured by radioimmunoassay (DiaSorin Inc, Stillwater MN USA). VDBP, Cathelicidin (LL-37) and β-defensin-2 levels were measured by ELISA using the following commercial kits: Quantikine Human Vitamin D Binding Protein (R&D Systems Europe, Abingdon, UK), HK321 Human LL-37 (Hycult Biotech, Uden, Netherlands) and human β-defensin-2 (Alpha Diagnostic International, San Antonio, TX, USA), respectively.
Statistical analysisCategorical and discrete variables were expressed as counts (percentage); continuous variables such as Vitamin D, VDBP, and related peptides were expressed as median and interquartile ranges. Statistical differences between groups were assessed with the Chi-square test, using Yates’ correction or Fisher's exact test as appropriate, for categorical variables. The Mann–Whitney U test was used for biomarkers, while the Student t-test was used for the rest of the continuous variables.
To compare the predictive value of Vitamin D, VDBP, and related peptides, receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was determined. To estimate the strength and independence of associations, Vitamin D, VDBP, and related peptides were divided into dichotomous variables according to median, and adjusted odds ratios (OR) with their 95% confidence interval (CI) for ‘in-hospital mortality’ were calculated using unconditional logistic regression.
The level of statistical significance was set at 0.05, and all tests were two-tailed. SPSS statistical software package 19.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
ResultsStudy populationSeventy-five septic shock patients were included in the study (53 men and 22 women) with a median age at admission of 64 years (Table 1). At ICU admission, median APACHE II, SOFA and SAPS II scores were 22, 10 and 42, respectively. Lungs were the most frequent source of infection. The ICU mortality rate was 24% and the hospital mortality rate was 28%. Non-survivors were more severely ill, as reflected by significantly higher severity scores and higher number of organ dysfunctions on ICU admission; mechanical ventilation and hemodiafiltration were also required in a higher percentage of cases compared to survivors. No significant differences were observed between hospital survivors and non-survivors with respect to serum 25(OH)D, 1,25(OH)2D, cathelicidin and defensin levels, or with respect to 25(OH)D sufficiency, deficiency or insufficiency.
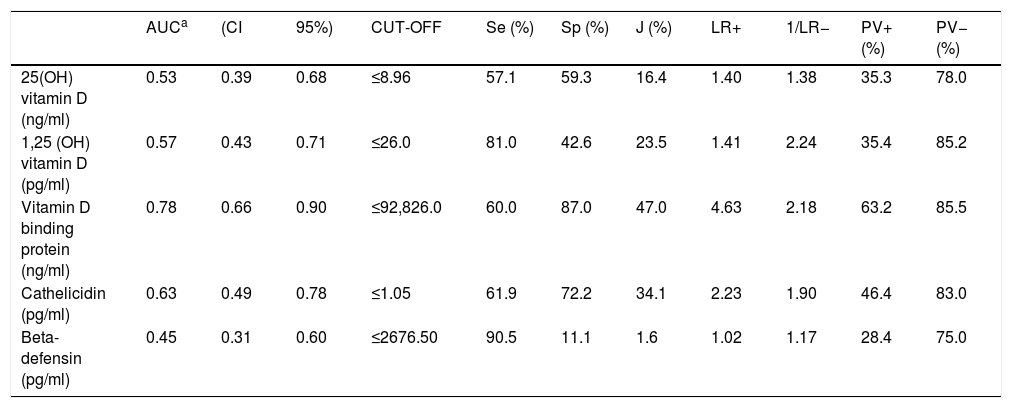
Among the prognostic factors studied, VDBP achieved the best rate for prediction of in-hospital mortality (AUC=0.78). The optimal cut-off point was set at 92,826ng/ml. According to this cut-off point, sensitivity was 60.00% and positive predictive value was 63.16%. Of the other measurements, cathelicidin (pg/mL) and 1,25(OH)2D (pg/mL) had the highest AUC values (0.63 and 0.57, respectively) (Table 2).
AUC and optimal cut-off points with their corresponding validity indexes and predictive values for VitD and related biomarkers in relation to ‘in hospital mortality’.
| AUCa | (CI | 95%) | CUT-OFF | Se (%) | Sp (%) | J (%) | LR+ | 1/LR− | PV+ (%) | PV− (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH) vitamin D (ng/ml) | 0.53 | 0.39 | 0.68 | ≤8.96 | 57.1 | 59.3 | 16.4 | 1.40 | 1.38 | 35.3 | 78.0 |
| 1,25 (OH) vitamin D (pg/ml) | 0.57 | 0.43 | 0.71 | ≤26.0 | 81.0 | 42.6 | 23.5 | 1.41 | 2.24 | 35.4 | 85.2 |
| Vitamin D binding protein (ng/ml) | 0.78 | 0.66 | 0.90 | ≤92,826.0 | 60.0 | 87.0 | 47.0 | 4.63 | 2.18 | 63.2 | 85.5 |
| Cathelicidin (pg/ml) | 0.63 | 0.49 | 0.78 | ≤1.05 | 61.9 | 72.2 | 34.1 | 2.23 | 1.90 | 46.4 | 83.0 |
| Beta-defensin (pg/ml) | 0.45 | 0.31 | 0.60 | ≤2676.50 | 90.5 | 11.1 | 1.6 | 1.02 | 1.17 | 28.4 | 75.0 |
AUC denotes Area Under Curve ROC for VitD and related biomarkers in relation to ‘in hospital mortality’ considering an inverse relation (the lower value of each biomarker, the higher risk of mortality). Cut-off denotes the optimal cut-off points considering as bad prognostic a value ≤the optimal value that maximizes the Youden index for a ratio ‘false negatives cost’/‘false positives’=3 and for the sample prevalence of ‘in-hospital mortality’. Se denotes Sensitivity. Sp denotes Specificity. J denotes Youden index. LR denotes Likelihood Ratios. PV denotes Predictive Values for sample prevalence (27.03%).
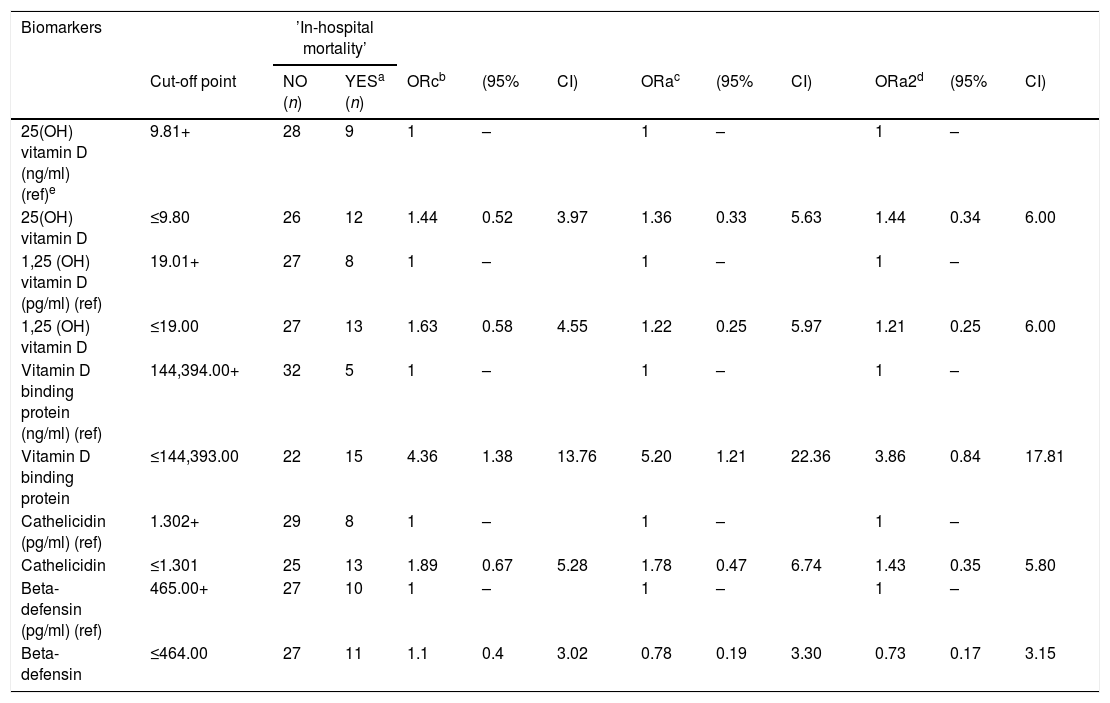
VDBP was the only variable that was statistically significantly associated with a higher risk of in-hospital mortality. This association was independent of the pre-established confounders (adjusted OR 5.20, 95% CI 1.21–22.36), p=0.027 (Table 3), although, when liver failure was added, the association decreased slightly (adjusted OR including also hepatic failure 3.86, 95% CI 0.84–17.81), p=0.083. When restricted to patients with vitamin D insufficiency (n=54), the predictive ability was rather similar (AUC=0.77), and an adjusted OR of 12.22 was found (95% CI 1.46–102.14), p=0.021 when adjusting for the pre-established confounders, that yielded statistical significance even including also hepatic failure as a confounding variable: adjusted OR including also hepatic failure. 9.46 (95% CI 1.04–85.47), p=0.046 (data not shown).
Crude and adjusted odds ratios (OR) for vitamin D, Vitamin D binding protein, and related peptides, in relation to ‘in hospital mortality’ in critically ill patients.
| Biomarkers | ’In-hospital mortality’ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off point | NO (n) | YESa (n) | ORcb | (95% | CI) | ORac | (95% | CI) | ORa2d | (95% | CI) | |
| 25(OH) vitamin D (ng/ml) (ref)e | 9.81+ | 28 | 9 | 1 | – | 1 | – | 1 | – | |||
| 25(OH) vitamin D | ≤9.80 | 26 | 12 | 1.44 | 0.52 | 3.97 | 1.36 | 0.33 | 5.63 | 1.44 | 0.34 | 6.00 |
| 1,25 (OH) vitamin D (pg/ml) (ref) | 19.01+ | 27 | 8 | 1 | – | 1 | – | 1 | – | |||
| 1,25 (OH) vitamin D | ≤19.00 | 27 | 13 | 1.63 | 0.58 | 4.55 | 1.22 | 0.25 | 5.97 | 1.21 | 0.25 | 6.00 |
| Vitamin D binding protein (ng/ml) (ref) | 144,394.00+ | 32 | 5 | 1 | – | 1 | – | 1 | – | |||
| Vitamin D binding protein | ≤144,393.00 | 22 | 15 | 4.36 | 1.38 | 13.76 | 5.20 | 1.21 | 22.36 | 3.86 | 0.84 | 17.81 |
| Cathelicidin (pg/ml) (ref) | 1.302+ | 29 | 8 | 1 | – | 1 | – | 1 | – | |||
| Cathelicidin | ≤1.301 | 25 | 13 | 1.89 | 0.67 | 5.28 | 1.78 | 0.47 | 6.74 | 1.43 | 0.35 | 5.80 |
| Beta-defensin (pg/ml) (ref) | 465.00+ | 27 | 10 | 1 | – | 1 | – | 1 | – | |||
| Beta-defensin | ≤464.00 | 27 | 11 | 1.1 | 0.4 | 3.02 | 0.78 | 0.19 | 3.30 | 0.73 | 0.17 | 3.15 |
OR refers to OR adjusted for 1,25 (OH) vitamin D, Vitamin D binding protein and Cathelicidin when appropriate (as continuous variables), sex, age, immunosuppression status, and Charlson index, Apache II score and total volume of fluid resuscitation during first 24 ICU hours as continuous variables.
Our results show that in patients with septic shock, VDBP values are better predictors of survival than vitamin D, cathelicidin and defensin. Thus VDBP values seem to have an inverse relation with respect to mortality, regardless of the vitamin D or vitamin D-related peptide levels.
In addition to transporting vitamin D3, VDBP has multifunctional properties: as well as macrophage activation, it enhances the leukocyte chemotactic activity of activated complement peptides and influences macrophage chemotaxis, and may also act through excessive globular actin scavenging.7,9,10 Thus, in critically-ill trauma patients, Dahl et al. found that low serum VDBP was associated with a higher risk of respiratory failure and sepsis development.11 Similarly, Jeng et al. compared a group of critically-ill subjects admitted to ICU with and without sepsis, and healthy controls. VDBP levels were significantly lower in subjects with sepsis compared to those without sepsis. This effect was not present for 25(OH)D and cathelicidin.12 In addition to these studies, our present study shows that, in septic shock patients, VDBP is a potential predictor of hospital mortality. Patients with low VDBP levels on admission to ICU had a six-fold higher risk of a poorer outcome. This association was not affected by the levels of other components of the vitamin D axis, or by the volume of fluid resuscitation before blood sampling. However, we must emphasize the effect that liver dysfunction presents on the association between VDBP and mortality. Although the strength of association between VDBP and in-hospital mortality (adjusted OR 3.45) remained the highest of all the studied factors, even higher than for the association of hepatic failure itself with mortality (adjusted OR 2.52), the significant association between VDBP and mortality was lost after adjusting by liver dysfunction, probably as a consequence of the limited sample size. This fact should be taken into account in future studies and in particular in clinical trials that about vitamin D supplementation in patients with sepsis are being carried out.
It has been shown that vitamin D status is associated with differential metabolic profiles during critical illness.13 In sepsis, in particular, it could be intimately associated with prognosis and vitamin D administration might be associated with a reduction in mortality.14,15 Braun et al.3 observed ICU preadmission 25(OH)D deficiency to be strongly associated with the risk of death in critical illness. They obtained 25(OH)D samples up to a year prior to ICU admission, potentially detracting from their predictive power at the time of critical illness. To resolve this limitation, the same group performed a follow-up study 4 that examined 25(OH)D levels around the time of critical care initiation and observed a similar association. The time span from measurement to ICU admission was relatively long (14 days). More recently, Parekh observed in both, clinical and murine experimental models, vitamin D deficiency to be associated with more severe cases of sepsis and higher mortality risk.16 In both cases, their analysis did not include information about the potential dilutional effect related to vigorous fluid resuscitation at the time of critical illness. In contrast, in our study, septic shock patients with decreased vitamin D levels demonstrated no pre-significant differences in hospital mortality. In our case, 25(OH)D levels were measured upon admission to the ICU, and results were adjusted for volume of fluid resuscitation in the patient. No differences were found for in-hospital mortality according to the 25(OH)D values. Evidently, these results may be affected by the small sample size. However, Barnett et al., in a population of 486 critically-ill patients, found no significant differences in in-hospital mortality either.17 Similarly, Azim et al. did not find a causal association between vitamin D deficiency and mortality.18 Finally, focused exclusively in critically ill patients with sepsis, both Nguyen et al. and Su et al. found no association between 25(OH)D serum levels and mortality.19,20 In our opinion, this discrepancy in results justifies a study that conclusively elucidates the impact of serum 25(OH)D at ICU admission on in-hospital mortality.
With respect to vitamin D related peptides, there is no doubt about the important role that both play in the immune response to the infection.21 However, their role in sepsis is still to be clarified. In our study, neither cathelicidin nor beta-defensin-2 levels predicted mortality, although there was a trend toward increased mortality with lower cathelicidin. The data in this regard are scarce but seem to follow the line of the results obtained in our study. Leaf et al. in 121 critically ill patients (14% with septic shock) observed low cathelicidin levels to be associated with 4 fold-higher risk of 90 day-mortality.22 Similarly, in the study published by Leow et al. in patients admitted with severe community acquired pneumonia lower values of serum cathelicidin showed a nonsignificant trend to an association with higher 30-day mortality.23 All these results appear to be consistent with research showing that cathelicidin supplementation is protective in murine models of sepsis24 but it requires more powerful studies to confirm this assumption.
Among the limitations of our study, it must be acknowledged that the generalizability of our findings is limited by the fact that this was a single center observational study with a small sample size. A second limitation is that we did not evaluate VDBP polymorphisms. These polymorphisms result in effects on both VDBP concentrations and functional consequences, including susceptibility to infection.25 Finally, our study lacks a control ICU group without sepsis to be compared with in order to obtain more conclusive results.
ConclusionsIn conclusion, based on our results, VDBP could be a marker of septic shock prognosis, with better accuracy than vitamin D and vitamin D-related antimicrobial peptides. Vitamin D and peptides are not associated with septic shock mortality.
Ethics approval and consent to participateThe study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the ethics committee of University Hospital Marqués de Valdecilla (CEIC Cantabria 2014.159).
Availability of data and materialsThe dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Author contributionsStudy concept and design: BS, MTGU, MS.
Acquisition of the data: BS, AFJ, BAL, SPSM.
Analysis and interpretation of the data: BS, MS.
Drafting of the manuscript: BS, MS.
Critical revision of the manuscript for important intellectual content: MTGU.
Statistical expertise: MS.
Authors’ informationNot applicable.
Consent for publicationNo individual patient data were reported.
Funding sourcesNone.
Competing interestThe authors declare that they have no competing interests.