Drug–drug interactions occur when one drug’s activity is modified by the action of another, either increasing or reducing its effect. Certain interactions can lead to therapeutic failure or cause toxicity, so it is necessary to identify them early to optimise treatment, thus guaranteeing its efficacy and safety.1
We present a clinical case in which a patient simultaneously received voriconazole and tamsulosin, an α-1A adrenoreceptor antagonist, with a clinically relevant drug-drug interaction being observed.
A 66-year-old male smoker and active drinker was admitted for constitutional syndrome, feelings of dysthermia and productive cough lasting two months. His medical history included hypertension, dyslipidaemia, Leriche syndrome, hyperuricaemia and residual pulmonary lesions due to having suffered from pulmonary tuberculosis at 17 and 25 years of age. In the initial physical examination, the patient was found to be cachexic and febrile, and on pulmonary ausculation presented hypophonesis in the left upper lobe.
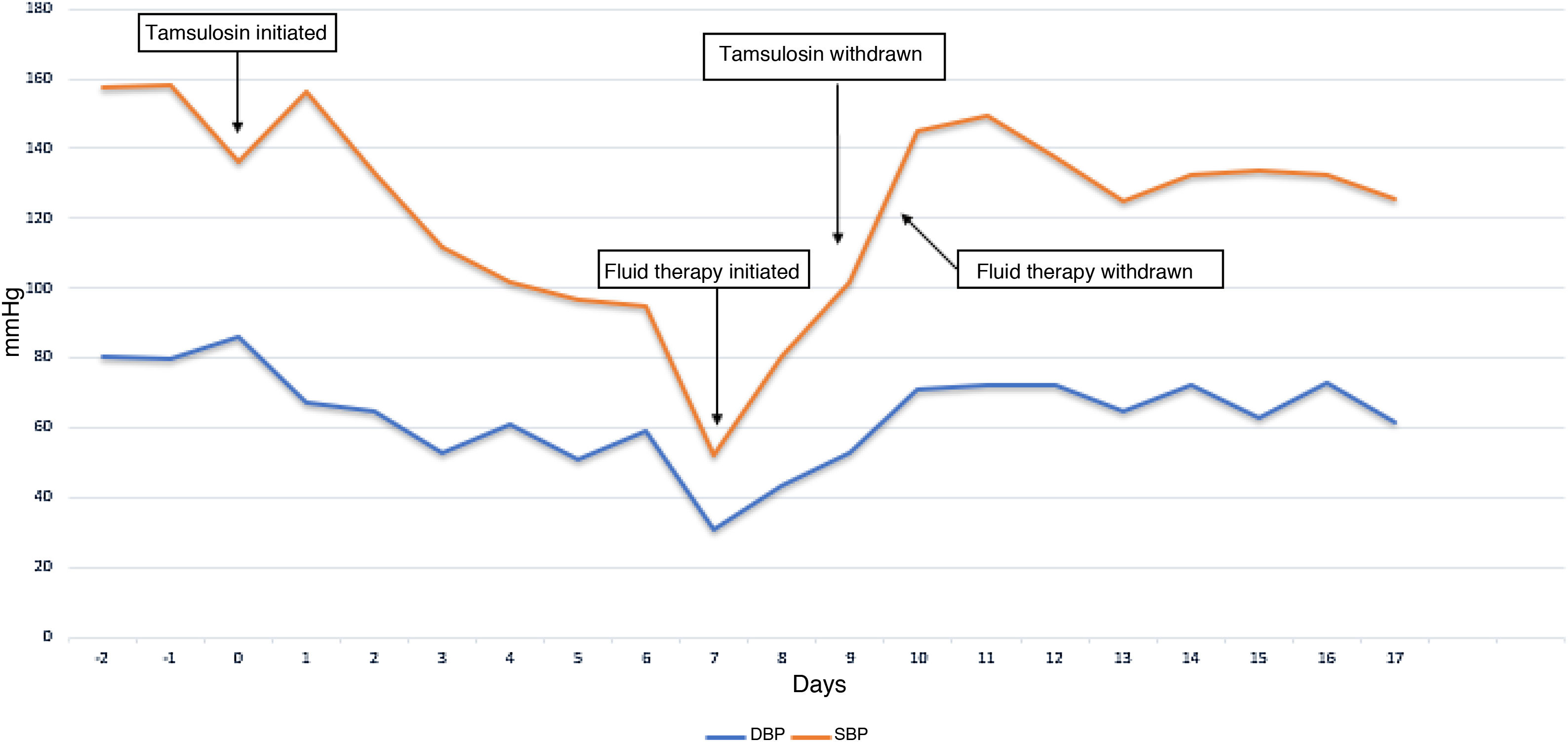
The chest X-ray and chest CT revealed multiple pulmonary bullae with invasion of the same and lung opacities predominantly on the left. Multiple sputum cultures were taken and found to be positive for Aspergillus fumigatus. Precipitins for A. fumigatus were also positive. In view of the clinical picture, together with the radiological and microbiological findings, it was treated as chronic cavitary bronchopulmonary aspergillosis, and treatment was initiated with voriconazole and nebulised amphotericin B. At two weeks from admission, the patient presented an episode of acute urinary retention, for which he required a urinary catheter and was started on tamsulosin, a drug that had never before been prescribed in this patient, to facilitate its management. Seven days after starting tamsulosin, he had an episode of sudden and symptomatic drop in blood pressure (BP), with systolic blood pressure values reaching 52 mmHg and diastolic values of 31 mmHg, with no evidence of hypovolaemia (no bleeding or dehydration) or alterations that would suggest cardiogenic or septic shock (with a review of the negative blood cultures). He was administered 500 ml of physiological serum, which increased his systolic blood pressure to 80 mmHg and diastolic blood pressure to 43 mmHg. He subsequently started fluid therapy with gradual improvement in BP (Fig. 1).
During the daily review of his treatment, after noting that there had been no recent changes in the antihypertensive treatment (he was on chronic treatment with 5 mg amlodipine), the possibility of a drug-drug interaction between voriconazole and tamsulosin was discerned. Simultaneous administration of the two drugs can trigger an increase in plasma concentrations of tamsulosin due to inhibition of its metabolism, accompanied by an increase in its pharmacological effect. In the case described, the pharmacological interaction manifested as a potentiation of tamsulosin's hipotensive effect. Consequently, the risk-benefit ratio of keeping tamsulosin in the pharmacotherapeutic plan was reviewed and it was ultimately decided to withdraw the drug. Over the following days, the patient's BP gradually increased and the supportive measures initiated could be withdrawn while maintaining control of BP within normal limits.
Tamsulosin is a substrate of cytochrome-P450 isoenzymes 2D6 (CYP2D6) and 3A4 (CYP3A4).2 Voriconazole is metabolised primarily by the isoenzymes CYP2C19 and, to a lesser extent, CYP3A4 and CYP2C9. It also has the potential to be an inhibitor of these three isoenzymes, and is considered a potent CYP3A4 inhibitor.3,4 It has been demonstrated that combined administration of tamsulosin with potent CYP2D6 inhibitors has only a limited effect on tamsulosin exposure, while with potent CYP3A4 inhibitors the effect is doubled.3,5,6 In this case, co-administration of tamsulosin and voriconazole was associated with haemodynamic changes (hypotension), deriving from a possible increase in the plasma concentration of tamsulosin. It should be noted that there are no published cases of this interaction in the literature. Nevertheless, without pharmacokinetic studies, the possibility that this was a “first dose phenomenon” associated with starting treatment with tamsulosin cannot be ruled out,7 even though in this case the clinical effect was observed seven days from initiation.
Taking into account the potential for drug-drug interaction between tamsulosin (substrate) and voriconazole (inhibitor) and the clinical relevance of a possible increase in the hypotensive activity of tamsulosin, the risk-benefit ration of administering the two drugs simultaneously would need to be adequately assessed, considering alternatives wherever possible.
Please cite this article as: Parramón-Teixidó CJ, Pau-Parra A, Burgos J, Campany D. Voriconazol y tamsulosina: interacción farmacológica clínicamente relevante. Enferm Infecc Microbiol Clin. 2021;39:361–363.