This quasi-experimental study compared the effectiveness of chewing gum and gargling with a saline solution as two types of intervention to reduce oral mucositis scores.
MethodThe sample consisted of 44 children who were divided into two groups, one of which chewed gum, and the other gargled with a saline solution. The Mann-Whitney U test was used to analyze the data.
ResultsThere was a significant difference (p = 0.001) in post-intervention oral-mucositis scores. The significant mean difference between the groups indicated that the decreased oral mucositis scores for the chewing gum group was more substantial than for the group gargling with a saline solution (p = 0.001).
ConclusionsThe data showed that chewing gum is more effective than gargling with a saline solution, and it can be incorporated into the nursing protocol for treating pediatric cancer patients.
Oral mucositis is a side effect of chemotherapy. The incidence of oral mucositis in pediatric cancer patients in the United States amounted to 132 000 cases1. At Indonesia's Sanglah Hospital Denpasar, based on a preliminary study conducted in two months period (January to February 2016), the data showed that 20 of 30 children undergoing chemotherapy experienced oral mucositis. It is known that chemotherapeutic agents can directly damage oral mucosa epithelial cells or compromise children's immune systems, leaving them vulnerable to infection. Although the mortality rate for oral mucositis is only 1%, 40% of patients experienced severe ulceration that caused physiological and functional disorders that decreased the patients’ quality of life2–4. A side effect with such an impact on quality of life requires proper management.
Management of oral mucositis in Sanglah Hospital Denpasar is still limited to oral debridement and oral decontamination (gargling with a saline solution). Oral debridement can be very traumatic and cause intense pain, risk of bleeding, and infection. Regarding oral decontamination, Sanglah Hospital uses three main ingredients, namely 0.2% chlorhexidine, iodine, and saline. However, some studies do not recommend the use of chlorhexidine and iodine because of their ineffectiveness in reducing the severity of oral mucositis5,6. Furthermore, neither of these substances should be used for a long period of time, because they interfere with the normal flora of mouth, and their alcohol content can cause dry mouth and irritate the tissues7.
Nurses, as professional health workers, have a vital role in preventing and managing oral mucositis in children undergoing chemotherapy. One treatment for resolving mucositis is the act of chewing gum, which increases both oral pH and saliva production. This can prevent xerostomia (dry mouth) and prevent or minimize irritation and ulceration. Gargling with a saline solution is also an option, because it is useful in maintaining oral mucosa moisture and accelerates tissue granulation.
The purpose of this study was to compare the effectiveness of chewing gum with that of gargling with a saline solution on resolving oral mucositis in children with cancer who are undergoing chemotherapy. The results of this study are expected to enrich pediatric nursing practice and directly benefit the treatment of oral mucositis in children undergoing chemotherapy.
MethodThe study employed a quasi-experimental design with consecutive sampling. The sample (n = 44) was composed of pediatric cancer patients ≥ 5 years old who were receiving chemotherapy. The children were divided into two groups of 22 each; one group used the chewing gum intervention, and the other was the saline-solution-gargling intervention group. The instrument used to measure oral mucositis scores was the Oral Assessment Guide (OAG)8, an instrument found by researchers to be valid and reliable9. Data collection was completed within a month. During the first two weeks of January 2016, we collected data from the saline-solution-gargling group, and the final two weeks of that month were devoted to collecting data from the gum-chewing group. Pre-test mucositis scores were recorded for the children prior to chemotherapy, with intervention-data collection beginning on the first day of chemotherapy and continuing until the sixth day for each group. Each intervention was administered three times daily, and all children fasted for one hour prior to engaging in their assigned intervention. Tooth-brushing was required prior to oral decontamination. Post-test mucositis scores were measured on the seventh day. Data were interpreted via univariate, bivariate, and multivariate analyses. Bivariate analysis used a non-parametric test, because the data were not normally distributed. Multivariate analysis was used to identify confounding variables and to learn the effects of the interventions after controlling confounding variables10.
ResultsAs shown on Table 1, the probability value is greater than 0.05, indicating that there are no significant differences in characteristics among the respondents in the full sample. This homogeneous data acquisition for all confounding variables has fulfilled one of the internal validity requirements for a research experiment, because it proves that the change in mucositis score did not occur because of variations in respondent characteristics; rather, the interventions were the cause.
Table 2 shows that there was no significant difference among mucositis scores before the interventions, indicating that all respondents in the study had similar characteristics and mucositis scores (p = 0.135; a = 0.05). Post-intervention mucositis scores revealed a significant difference after the gum-chewing and the gargling interventions (p = 0.029; α =0.05).
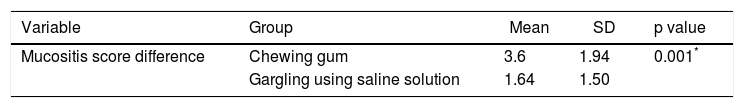
Table 3 shows a decline in oral mucositis scores in both the gum-chewing group (3.6) and the saline-solution-gargling group (1.64). However, the decrease in mucositis score is greater in the gum-chewing group (p = 0.001; a = 0.05), indicating that chewing gum is a more effective intervention to reduce oral mucositis than is gargling with a saline solution.
Oral mucositis score decreases.
| Variable | Group | Mean | SD | p value |
|---|---|---|---|---|
| Mucositis score difference | Chewing gum | 3.6 | 1.94 | 0.001* |
| Gargling using saline solution | 1.64 | 1.50 |
Based on independent t-test data, the following respondent characteristics were associated with patient oral mucositis scores: age, oral fluid intake, whether receiving the therapeutic combination, and nutritional status. Multivariate analysis using linear regression showed that the age variable is the most significant influence on oral mucositis scores in both groups: chewing gum (p = 0.015; α = 0.05) and gargling with a saline solution (p < 0.001; α = 0.05).
DiscussionBased on Levine's Nursing Conservation Model Theory, children with cancer who are undergoing chemotherapy should be seen as individuals adapting to threats from internal and external environments. Levine also states that nursing care focuses on helping patients preserve structural integrity which, in these cases, pertains to the oral mucosa11. Two therapeutic interventions for regaining oral-mucosa structural integrity are gargling with a saline solution and chewing gum11.
Analysis of the study's results revealed a significant difference in post-intervention mucositis scores between the two groups. This finding is in line with another study that found the sweets in gum being conducive to increasing saliva production and saliva pH to prevent the occurrence of xerostomia (dry mouth) due to chemotherapy12. The saline-solution-gargling intervention also decreased children's oral mucositis scores due to the saline solution's mechanism, which acts to maintain the moisture of oral mucosa. Gargling activity can also remove the chemotherapeutic-agent residue that remains in the epithelium or salivary gland, thereby preventing direct contact of chemotherapeutic agents with oral mucosa13. Thus, both of these interventions can reduce the severity of oral mucositis in children.
The differing decreases in mucositis score indicates that chewing gum is more effective than gargling with a saline solution. While there is no previous literature confirming that children prefer the chewing gum intervention over the saline-solution-gargling intervention, the authors of this study have made assumptions based on experience, observation, and interviews with children and parents. We believe the reason behind this preference for gum chewing is that gum is usually quite tasty and attractively colored, while saline solution tastes rather salty. Hence, because children preferred the chewing gum intervention, they were more eager and motivated to brush their teeth, which was a required activity prior to engaging in the intervention. This increased willingness not only has a beneficial impact on improving the children's oral hygiene but, importantly, it reduces the risk of infection occurrence due to gram-negative bacteria or fungi4. Gargling with a saline solution did not provide that incentive and, in fact, children tended to need to be forced to participate in gargling interventions, and they looked uncomfortable while doing so. Discomfort can increase stress in children, which then stimulates an increase in the concentration of cortisol in both the saliva or blood circulation14. Cortisol can weaken a patient's immune system, raise the blood-glucose levels that cause an increase in hemoconcentration and, eventually, reduce the transport of oxygen to tissues15. That process can cause hypoxia and tissue damage which, in turn, can become a microbial growth medium that leads to oral mucositis or prolong the oral-mucositis healing process.
Saliva components are also thought to contribute to differences in mucositis scores due to the organic contents of saliva are lysozyme, lactoperoxidase, proline-rich protein, and mucin. Lysozyme and lactoperoxidase help inhibit bacteria growth and kill bacteria16,17. Proline-rich proteins form new tissue in oral mucosal epithelial tissue. Mucin protects mucosa from dehydration and acts as a buffer system that prevents bacterial colonization and cleans acid substances resulting from bacterial metabolism. In addition to its organic components, saliva also has an inorganic composition that resembles saline solution, which consists of sodium and chloride16,17. Thus, the mechanism of NaCl, which can be found in saliva during chewing gum, in maintaining oral hygiene is similar to the act of chewing gum. This is why chewing gum is thought to be more effective in reducing oral mucositis score of children with cancer.
The analysis of the role of respondent characteristics showed that the variables of age, oral fluid intake, whether receiving a therapeutic combination, and nutritional status are associated with patients’ oral mucositis scores. However, age is the most dominant variable: the younger the child's age, the higher his or her oral mucositis score. Two previous studies found that the child population has a higher oral-mucositis risk because their epithelial-tissue cell proliferation is more rapid than in adults4,18. The literature also shows that children's epithelial cells are more sensitive to toxicity8. All of these risk factors cause children to tend to need higher doses of chemotherapy4. Volpato et al. described that therapeutic dosage level and whether patients are undergoing combination therapy affect the severity of oral mucositis19. Combination therapy for these children is a course of treatment composed of chemotherapy along with radiotherapy of the head and neck areas. Of the 5 children in this study receiving combination therapy, 4 were between 5 and 9 years old. Radiotherapy in these areas has been shown to cause several complications, including dry mouth and oral mucositis20. This susceptibility is caused by the emission of radiotherapy electrons, which may lead to the soft oral mucosa membranes becoming dry and brittle, possibly resulting in ulceration and injury. This is also supported by results in the literature showing that head and neck cancer patients undergoing radiotherapy had a 60% risk of developing oral mucositis, and that more than 90% of oral mucositis cases occurred in patients receiving radiotherapy and chemotherapy simultaneously21,22. Further confirmation of these findings was seen in a study revealing that 80% of children who undergo a combination of chemotherapy and head and neck radiotherapy had an 80% risk of developing oral mucositis23.
Younger ages in children appeared to be an additional complication, as younger children tend to be uncooperative about some intervention protocols. Based on observations and interviews conducted by researchers during our study, higher oral-mucositis scores in children aged 5-7 years were caused by their reluctance to perform oral hygiene, such as voluntary tooth-brushing or gargling. In fact, according to the parents, some children had to be forced to brush their teeth. According to reporting from the parents, on previous occasions when their children experienced chemotherapyrelated oral mucositis, they refused to perform oral care in any form. Such behaviors can promote development of microorganisms in the mouth that will further aggravate oral mucositis conditions, particularly in the ulcerative phase, when colonization of gram-negative bacteria and fungi can damage mucosa surfaces that are no longer intact. The situation will be exacerbated if patients experience neutropenia, as the infection process will last longer and be more widespread24,25.
Another relevant phenomenon is that younger children are more likely to refuse to eat while experiencing mucositis, which is attributable to pain and not being able to taste food. The taste deficit is a result the papillae on patients’ tongues being covered by pseudomembranous, which can disrupt adequate nutritional intake. This reaction is in accordance with our study's findings: of 6 children with abnormal nutritional status, 4 were aged 5-9 years old. As explained before, adequate nutritional intake, especially protein intake, is needed to repair damaged tissues26.
Based on the results of multivariate analysis (in addition to age), receiving a therapeutic combination also affected oral mucositis scores in the chewing gum intervention group. In theory, children who undergo the therapeutic combination had a higher risk of experiencing oral mucositis or of suffering a more severe case. However, in this study, children in the gum-chewing group who were undergoing a therapeutic combination experienced a decrease in mucositis score. This showed that chewing gum is effective for reducing the severity of mucositis, especially in children who undergo a therapeutic combination.
This study has several implications, especially for pediatric nursing of children with chronic illnesses, and for expanding knowledge regarding independent interventions that can be provided to children with cancer who undergo chemotherapy, regardless of whether they suffer from oral mucositis. Nurses can also provide health education to patients and families about the chewing gum regimen. The use of chewing gum as an intervention can reduce the need for painful interventions such as oral debridement. This intervention comports with pediatric, traumatic-care, and family-centered-care nursing principles. We expect this research to be the basis for further study.
Special thanks are extended to the director and head of nurses at Sanglah Hospital, and to the research assistants who were involved in the data collection process. In addition, we thank the pediatric cancer patients and their parents for serving as the respondents in this study.