To assess the effectiveness of the implementation of a protocol for glycaemic control in critical care, in terms of maintenance of a pre-established target of blood glucose level, reduction of hyperglycaemia and prevention of severe hypoglycaemia.
MethodProspective “pre-post” quasi-experimental study carried out in a general critical care unit. Adult patients treated with intravenous insulin were included. We recorded all glycaemic tests performed from November 2014 to August 2015 (pre-intervention) and from November 2015 to August 2016 (post-intervention). The intervention consisted of the implementation of an evidence-based glycaemic control protocol to achieve glycaemic levels in a range of 140–180mg/dl. Main variables analysed were: proportion of glycaemic tests in the target range, proportions of severe hypoglycaemia (under 40mg/dl) and hyperglycaemia over 200mg/dl.
ResultsWe analysed 7864 glycaemic tests from 125 patients, 66 pre-intervention and 59 post-intervention. Average age was 66.24±13.99 years, 64% of patients were male. The proportion of tests within the target range was higher in the intervention group (38.82 vs. 44.34 p<.001). Only one case of severe hypoglycaemia was identified, which happened in the pre-intervention period. The rate of severe hyperglycaemia was lower in the post-intervention group (19.19 vs. 16.28 p=.001).
ConclusionsOur experience shows that implementation of evidence-based interventions can improve glycaemic control during critical illness. We found higher glycaemia levels in the target range. The protocol proved useful in the prevention of severe hypoglycaemia. Nurse-led interventions based on clinical data improved health results in our patients.
Evaluar la efectividad de la implantación de un protocolo de control glucémico del paciente crítico liderado por enfermeras, en términos de mantenimiento de un rango preestablecido de glucemias, reducción de hiperglucemia y prevención de hipoglucemia severa.
MétodoEstudio cuasiexperimental prospectivo «pre-pos» realizado en una unidad de cuidados intensivos polivalente. Se incluyeron pacientes adultos en tratamiento con insulina endovenosa. Se registraron todas las determinaciones de glucemia desde noviembre de 2014 a agosto de 2015 (preintervención) y desde noviembre de 2015 a agosto de 2016 (pos). La intervención consistió en la implementación de un protocolo basado en la evidencia, para conseguir rangos de glucemia entre 140-180mg/dl. Las variables principales incluyeron proporciones de glucemias dentro de rango, tasas de hipoglucemia severa (menos de 40mg/dl) y tasas de hiperglucemia superior a 200mg/dl.
ResultadosSe evaluaron 7864 determinaciones de glucemia pertenecientes a 125 pacientes, 66 preintervención y 59 postintervención. La edad media fue de 66,24±13,99 años, el 64% eran hombres. La proporción de determinaciones dentro del rango fue superior en el grupo postintervención (38,82 vs. 44,34 p<0,001). Se identificó un caso de hipoglucemia severa, que sucedió en el grupo preintervención. La tasa de hiperglucemia severa resultó menor en el grupo postintervención (19,19 vs. 16,28 p=0,001).
ConclusionesNuestra experiencia muestra que una implantación basada en la evidencia puede mejorar el control glucémico en pacientes críticos. Se observaron mayores tasas de glucemia dentro de rango. El protocolo resultó útil en la prevención de la hipoglucemia severa. El liderazgo del equipo de enfermería y la toma de decisiones autónomas basadas en datos clínicos permitió mejorar los resultados en salud de los pacientes.
Hyperglycaemia in critical patients has been associated with various adverse effects and increased morbidity in intensive care units. The control of hyperglycaemia poses a challenge for intensive care professionals. Intravenous insulin infusion requires strict monitoring which can be complex due to the risk of severe hypoglycaemia and its direct relationship with the mortality of critically-ill patients.
The implementation of nurse-led protocols has improved care practice and the clinical safety of critically-ill patients receiving intravenous insulin. Our study provides information on improvements in glycaemia control introduced via an evidence-based implementation and care-practice normalisation strategy led by the nursing team. The role of nurses in the continuous monitoring of glycaemia and correct dosage of insulin therapy is essential for the safe administration of insulin, ensuring that the therapy adapts better to a changing clinical situation.
Implications of the study?Our experience could help to improve action protocols and promote the implementation of similar actions that reduce clinical variability and improve glycaemia control for patients. It shows that the leadership of nurses in a multidisciplinary intervention and their autonomy in decision-making can result in more effective glycaemia control. The study could be useful for healthcare professionals and managers in the area of critical care committed to continuous improvement. It could also prove useful for researchers interested in the implementation of evidence in the clinical environment.
Stress-induced hyperglycaemia, also known as hyperglycaemia in critically-ill patients or stress-induced diabetes, is a common event in patients receiving care in intensive care units (ICU), with an incidence of between 30% and 50%.1,2 In general, stress-induced hyperglycaemia is understood as the onset of hyperglycaemia maintained at above 200mg/dl during the critical illness, irrespective of whether or not the patient had diabetes mellitus beforehand. Although the causative mechanisms have not been fully clarified, increased levels of cortisol and other counter regulatory hormones have been proposed as possible causes.1,3 Another possibly related factor is insulin resistance, which can have an incidence of up to 80% in the critically-ill.4
Different studies have associated maintained hyperglycaemia with increased mortality and other unwanted outcomes,5–7 including increased hospital stay, increased stay in ICU and a higher incidence of hospital-acquired infections. Given that most of the available evidence comes from observational studies, it is difficult to establish whether hyperglycaemia is the cause of the increase in mortality or a marker associated with the severity of a specific pathological process. Some investigators even suggest that a certain tendency towards hyperglycaemia could be a protective factor in some diseases or patient profiles.8 In any case, most clinical practice guidelines used in our environment agree that uncontrolled hyperglycaemia has potentially harmful effects and should be prevented.9–11 Actions to reduce hyperglycaemia have resulted in reduced mortality in some studies, although not in all.12
This situation has led to the development of protocols for the strict control of glycaemia in critically- ill patients, referred to as intensive insulin therapy protocols13–17 in some publications, with the aim of maintaining continuous levels of physiological glycaemia between 80 and 110mg/dl, using short-term intravenous (IV) insulin infusions. Various clinical trials have shown, however, that management of these ranges of glycaemia can prove complex,18–20 principally due to the higher incidence of severe hypoglycaemia (glycaemia below 40mg/dl) that reaches 19%, and moderate hypoglycaemia (glycaemia below 80mg/dl) with incidences of up to 32%. Furthermore, these protocols require more frequent glycaemia tests and extremely rigorous monitoring. The abovementioned trials coincide in that they relate the onset of severe hypoglycaemia with increased mortality, which by itself is a risk factor for death, irrespective of diabetes, the severity of the primary disease, age, sepsis or the use of mechanical ventilation.21–23
For these reasons, there is currently much scientific debate as to the target range of glycaemia levels in critically-ill patients. For the most part, the scientific community consider maintained hyperglycaemia an unwanted effect, but intensive insulin therapy protocols report risks that have not yet been fully controlled. Therefore the use of protocols with safer ranges in preventing hypoglycaemia is becoming more widespread; the most frequent benchmark levels are: 110–150mg/dl, or 140–180mg/dl.24 There has been increasing interest from practitioners who care for the critically ill in the development and implementation of reliable glycaemia control protocols agreed by health teams. Combining criteria and the protocolisation of glycaemic control with a view to reducing the incidence of severe hypoglycaemia could reduce the risk of death in patients who require intensive care.25
In recent years, various tools and protocols of varying efficacy have been tested. In general, computer tools have proved more capable and agile in maintaining appropriate ranges of glycaemia and in reducing adverse events.26 However rigorous control of glucose levels requires consideration of very diverse variables, including control of nutritional support; provision of carbohydrates, IV, in dilution or in drugs; the effect of some drugs on glycaemia or on insulin; the interference of diagnostic tests and therapeutic procedures in administering nutrition, insulin or other drugs; the use of contrast media that can require interruptions to nutritional support and other clinical situations.27 In addition, some technical aspects related to the activation of IV insulin should be taken into account, such as the need for uniform dilutions and concentrations, or compatibility with drugs infused into the same lumen. Finally, it is essential to ensure that the measures for taking blood samples for glycaemia testing are valid ensuring that all tests are equivalent to each other. All these factors can affect the maintenance of target levels of glycaemia, and all these variables are difficult to manage with one single tool.27,28
To respond to these many factors, nurse-led protocols have focussed on training, raising the awareness of and strengthening the nursing team in decision-making and managing complex clinical situations to maintain target glycaemia levels, generally supported by computer tools or insulin dosage calculators.12,29
This study seeks to evaluate the effectiveness of implementing a protocol for glycaemic control in ICU, in terms of maintaining pre-established target glycaemia levels (140–180mg/dl) and preventing severe hypoglycaemia. We also sought to assess its impact on preventing hyperglycaemia above 200mg/dl (severe hyperglycaemia).
MethodA prospective, “pre-post” quasi-experimental study carried out in a general ICU of a regional hospital of the Balearic Islands. The unit has 6 cubicles and attends more than 200 critically ill patients annually with over 80% occupation. There are 3 permanent nursing staff and 3 nursing auxiliaries, with a ratio of 2 patients per nurse over 24h. The medical team is comprised entirely of intensive care doctors, 3 doctors on the morning shift (08.00 to 15.00), and one doctor for the rest of the day.
The intervention comprised the design and implementation of an evidence-based protocol to achieve target glycaemia ranges between 140 and 180mg/dl, supported using a public-access computer tool available at http://rccc.eu/protocolos/HG/index.html. This tool is based on the algorithm published by the NICE-SUGAR group in 2004, which serves as a benchmark to calculate doses of insulin therapy. This protocol falls within a set of actions to improve clinical safety in intensive care in our centre. The intervention was undertaken between September and November 2015.
We undertook structured literature searches databases and meta search engines for health sciences to design the protocol: EBSCO-HOST (Medline, CINHAL and Cochrane), PubMed, Embase, CuidenPlus and Guiasalud, using the terms “insulin (insulin)”, “terapia con insulin (insulin therapy)”, “cuidados críticos (critical care)”, “guía (guideline)” and “protocolo (protocol)”, and the equivalents in English from the Medical Subject Headings (MeSH) vocabulary. Research studies of any methodology were chosen whose objectives concerned the administration of IV insulin (comparison of therapeutic alternatives, protocols and guidelines, drug compatibility and interactions, among others). We prepared descriptive tables with the main results of each study.
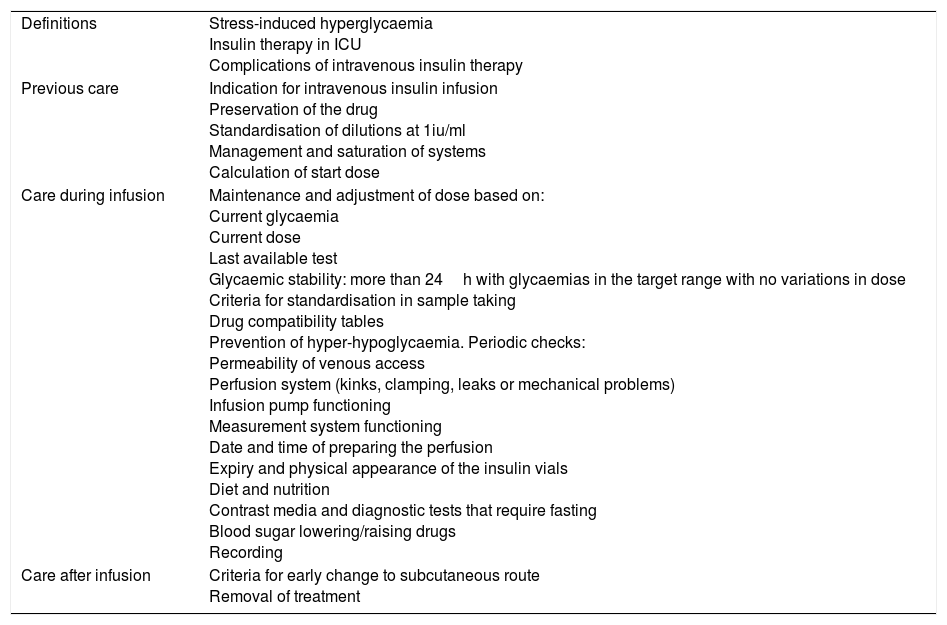
More than 50 activities and recommendations were agreed taking an interdisciplinary approach that included the preparation of the infusion, preservation, periodic substitution of systems, dosage calculation algorithms, drug compatibility tables, criteria for uniformity in sample taking, criteria for nurse decision-making, interrelationships with diet therapy protocols and actions to prevent complications, setting the maintenance of glycaemia levels between 140mg and 180mg/dl as the outcome yardstick. Four nurses, 2 intensive care doctors and a pharmacist took part in this process. The actions agreed in the protocol were later reviewed by the ICU patient safety team. The final version was validated by the hospital quality care commission. Table 1 provides a summary of the most relevant content included in this definitive version.
Summary of the contents of the care protocol.
| Definitions | Stress-induced hyperglycaemia Insulin therapy in ICU Complications of intravenous insulin therapy |
| Previous care | Indication for intravenous insulin infusion Preservation of the drug Standardisation of dilutions at 1iu/ml Management and saturation of systems Calculation of start dose |
| Care during infusion | Maintenance and adjustment of dose based on: Current glycaemia Current dose Last available test Glycaemic stability: more than 24h with glycaemias in the target range with no variations in dose Criteria for standardisation in sample taking Drug compatibility tables Prevention of hyper-hypoglycaemia. Periodic checks: Permeability of venous access Perfusion system (kinks, clamping, leaks or mechanical problems) Infusion pump functioning Measurement system functioning Date and time of preparing the perfusion Expiry and physical appearance of the insulin vials Diet and nutrition Contrast media and diagnostic tests that require fasting Blood sugar lowering/raising drugs Recording |
| Care after infusion | Criteria for early change to subcutaneous route Removal of treatment |
The implementation process followed the model described in the implementation guidelines of the Registered Nurses’ Association of Ontario,30 which has been used in various international settings. Multidisciplinary training sessions were held, accompaniment of professionals in practice, and follow-up of complex or doubtful cases. The healthcare professionals were also given a group email address and a mailbox was placed for anonymous collection of opinions from the healthcare team. Relevant opinions were used to include changes or corrections in the protocol. The implementation actions were led by nurses and developed in a multidisciplinary team.
Putting the protocol into operation implied that all interventions and decisions deriving from IV insulin infusion, except for the prescription to start the infusion, were undertaken by the nurse in charge of the patient. If necessary, the nurse could consult the intensive care doctor or pharmacy department.
All the adult patients who required IV insulin treatment for hyperglycaemia in the context of critical illness of any origin were included consecutively for the evaluation, the onset of hyperglycaemia above 180mg/dl in at least 2 independent tests was required. Patients taking an oral diet or enteral feeding at stable doses were excluded, since they were candidates for early subcutaneous insulinisation.
The clinical trial published by Praiser in 2009,20 was used as the benchmark to calculate the sample size, which in one of its arms used the same target glycaemia range that we proposed for our protocol, resulting in an incidence of severe hypoglycaemia of 2.7%. Thus, accepting an alpha risk of .05 and a beta risk of .2 in a bilateral contrast, a minimum of 43 subjects in each phase were required to detect the difference between two proportions as statistically significant, which for group 1 was expected to be .27, and .05 for group 2. A 10% rate of losses to follow-up was estimated. We used the arc sine approximation. A retrospective review of the nursing records between the months of March and June 2014 was performed to estimate the observation time necessary.
Demographic variables (age and sex) were assessed, as well as various conditions of the health process:
Reason for admission grouped according to the diagnostic category of the International Classification of Diseases (ICD-10).
Reason for discharge.
Length of hospital stay.
Severity scales of the process of critical illness: Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score (SAPS III).
Variables of the pathological process potentially associated with hyperglycaemia: shock of any origin, recent surgery (immediate post-operative period), sepsis or severe infection and metabolic disease.
The glycaemia levels were recorded from all the blood glucose tests performed on each patient included in the sample from November 2014 to August 2015 (pre-intervention) and from November 2015 to August 2016 (post- intervention). The samples were taken from the venous or arterial catheter after discarding between 4ml and 5ml of blood. Capillary samples from patients were also included where it was confirmed that the difference compared to the venous samples was less than ±10mg/dl. The protocol design was started in the pre-intervention phase and the diffusion and implementation actions were undertaken progressively between May and November 2015. The computer tool to support calculation of dose was used from the start of the observation, before the pre-intervention phase.
The data were taken from the patients’ computerised clinical histories, where the nurses record their activity in each work shift. The information was progressively downloaded to a Microsoft Excel spreadsheet for subsequent analysis.
The main variables for analysis included the proportions of glycaemic tests within range, rates of severe hypoglycaemia (below 40mg/dl) and ranges of hyperglycaemia above 200mg/dl, following the criteria of the benchmark guidelines.31
Epidat 4.1 software was used for the data exploitation. A descriptive analysis of all the study variables was performed, using mean and standard deviation for continuous variables and frequencies and percentages for the categorical variables. The Kolmogorov-Smirnov test was used to check the normal distribution of continuous variables. The comparison between the study periods was performed using the Student's t-test for continuous variables, and χ2 for nominal variables. Statistical significance was established with p values below .05.
The project was approved by the hospital's research committee. The authors have no conflicts of interest to declare.
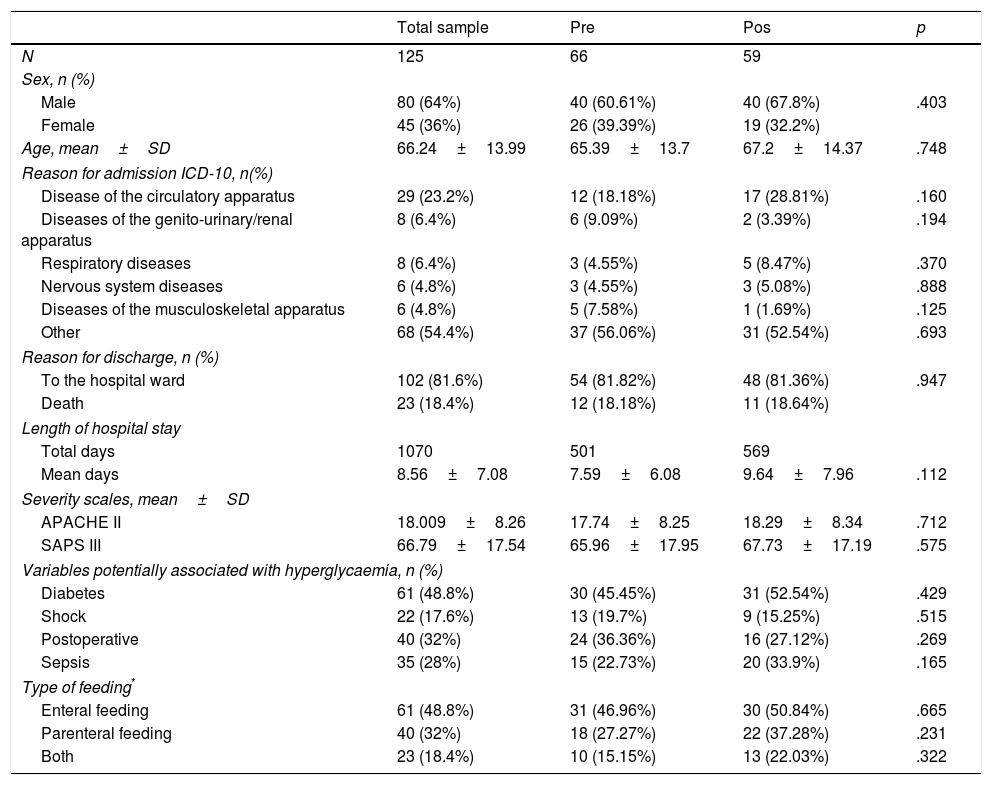
ResultsWe evaluated 7864 glycaemia tests from 125 patients, 66 pre-intervention, and 59 post-intervention. The pre- and post-intervention groups were homogeneous. No significant differences were identified in terms of the severity of the critical illness or the incidence of circumstances potentially related to the onset of stress-induced hyperglycaemia. The distribution of age, sex, diabetes, shock and other adjustment variables was similar in both groups (Table 2).
Patient features.
| Total sample | Pre | Pos | p | |
|---|---|---|---|---|
| N | 125 | 66 | 59 | |
| Sex, n (%) | ||||
| Male | 80 (64%) | 40 (60.61%) | 40 (67.8%) | .403 |
| Female | 45 (36%) | 26 (39.39%) | 19 (32.2%) | |
| Age, mean±SD | 66.24±13.99 | 65.39±13.7 | 67.2±14.37 | .748 |
| Reason for admission ICD-10, n(%) | ||||
| Disease of the circulatory apparatus | 29 (23.2%) | 12 (18.18%) | 17 (28.81%) | .160 |
| Diseases of the genito-urinary/renal apparatus | 8 (6.4%) | 6 (9.09%) | 2 (3.39%) | .194 |
| Respiratory diseases | 8 (6.4%) | 3 (4.55%) | 5 (8.47%) | .370 |
| Nervous system diseases | 6 (4.8%) | 3 (4.55%) | 3 (5.08%) | .888 |
| Diseases of the musculoskeletal apparatus | 6 (4.8%) | 5 (7.58%) | 1 (1.69%) | .125 |
| Other | 68 (54.4%) | 37 (56.06%) | 31 (52.54%) | .693 |
| Reason for discharge, n (%) | ||||
| To the hospital ward | 102 (81.6%) | 54 (81.82%) | 48 (81.36%) | .947 |
| Death | 23 (18.4%) | 12 (18.18%) | 11 (18.64%) | |
| Length of hospital stay | ||||
| Total days | 1070 | 501 | 569 | |
| Mean days | 8.56±7.08 | 7.59±6.08 | 9.64±7.96 | .112 |
| Severity scales, mean±SD | ||||
| APACHE II | 18.009±8.26 | 17.74±8.25 | 18.29±8.34 | .712 |
| SAPS III | 66.79±17.54 | 65.96±17.95 | 67.73±17.19 | .575 |
| Variables potentially associated with hyperglycaemia, n (%) | ||||
| Diabetes | 61 (48.8%) | 30 (45.45%) | 31 (52.54%) | .429 |
| Shock | 22 (17.6%) | 13 (19.7%) | 9 (15.25%) | .515 |
| Postoperative | 40 (32%) | 24 (36.36%) | 16 (27.12%) | .269 |
| Sepsis | 35 (28%) | 15 (22.73%) | 20 (33.9%) | .165 |
| Type of feeding* | ||||
| Enteral feeding | 61 (48.8%) | 31 (46.96%) | 30 (50.84%) | .665 |
| Parenteral feeding | 40 (32%) | 18 (27.27%) | 22 (37.28%) | .231 |
| Both | 23 (18.4%) | 10 (15.15%) | 13 (22.03%) | .322 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; SD: standard deviation; SAPS III: Simplified Acute Physiology Score.
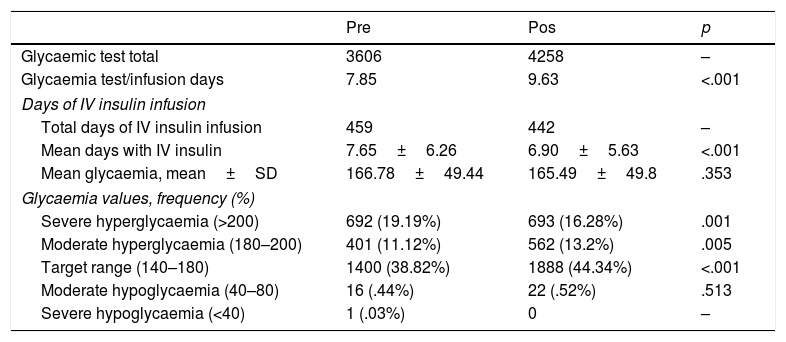
Only one case of severe hypoglycaemia was identified, which occurred in the pre-intervention group and did not cause observable effects in the patient. The proportion of tests within the target range was higher in the post-intervention group (38.82 vs 44.34, p<.001). The rate of severe hyperglycaemia was also lower in the post-intervention group (19.19 vs 16.28 p=.001). We also observed a non-significant reduction in mean glycaemia after the intervention (166.78±49.44 vs 165.49±49.8). The number of daily tests undertaken for glycaemic monitoring was higher in the post-intervention group. Table 3 shows the grouped results of the glycaemic tests in both periods.
Results of glycaemic control.
| Pre | Pos | p | |
|---|---|---|---|
| Glycaemic test total | 3606 | 4258 | – |
| Glycaemia test/infusion days | 7.85 | 9.63 | <.001 |
| Days of IV insulin infusion | |||
| Total days of IV insulin infusion | 459 | 442 | – |
| Mean days with IV insulin | 7.65±6.26 | 6.90±5.63 | <.001 |
| Mean glycaemia, mean±SD | 166.78±49.44 | 165.49±49.8 | .353 |
| Glycaemia values, frequency (%) | |||
| Severe hyperglycaemia (>200) | 692 (19.19%) | 693 (16.28%) | .001 |
| Moderate hyperglycaemia (180–200) | 401 (11.12%) | 562 (13.2%) | .005 |
| Target range (140–180) | 1400 (38.82%) | 1888 (44.34%) | <.001 |
| Moderate hypoglycaemia (40–80) | 16 (.44%) | 22 (.52%) | .513 |
| Severe hypoglycaemia (<40) | 1 (.03%) | 0 | – |
SD: standard deviation; IV: intravenous.
Our experience shows that an intervention to implement evidence-based recommendations can be effective in improving glycaemic control in critically-ill patients who require IV insulin.
The rate of hypoglycaemia that we observed was very much lower than previous studies in both observation periods since the only case we observed occurred in the pre-intervention phase and accounted for .03% incidence in this group, very much below the majority of published series.18,20,32 The computer tool was useful in preventing the main complication in these patients and an independent cause of mortality. We understand that the efficacy of this tool in preventing severe hypoglycaemia did not depend solely on the implementation process, but also in the use of a target range from 140mg/dl to 180mg/dl, which was far from the risk values, allowing a greater margin for the prevention of hypoglycaemias and therefore proving safer. The risks attributable to hypoglycaemia secondary to treatment with IV insulin could be reduced by implementing appropriate therapeutic algorithms, minimising monitoring errors and preventing the over-correction of hypoglycaemia.
The improvement obtained in the rates of glycaemia within the range were very similar to a previous study published in 2011 by Khalaila et al.,33 that showed an improvement in target glycaemia of 10.2% using a target glycaemia range of 110–149mg/dl. Other previous studies achieved higher rates of glycaemia within the target range, although there was also a higher onset of severe hypoglycaemia.29,34,35 This points to the need to undertake very frequent glycaemic controls that enable constant adjustments in dose. Thus, glycaemic control protocols should be sufficiently flexible to be really safe in preventing severe hypoglycaemias. In our case, we chose to implement means to increase patient safety in order to set out, for the future, actions to improve their efficacy and maintenance through time.
Our protocol implied a significant increase in the controls undertaken, which could be understood as a marker of the adherence by clinical nurses to the procedure. Nonetheless, fewer controls were undertaken than those of most previous studies.20,27,36 This situation might be due to the measures we included in our protocol to prevent unnecessary controls that lead in turn to manipulation and added risks. Therefore, in the event of glycaemic stability maintained over more than 24h, the time between controls could be increased to 6h if there were no changes in glycaemic intake via nutrition or fluid replacement, administration of contrast media, changes of drug doses that interact with insulin or changes in the patient's general condition and treatment in progress. Thus, our protocol implies an improvement in patient safety and provides greater legal safety in clinical decision-making by nurse caregivers.
Multidisciplinary consensus was crucial in driving some of the measures included in the protocol. Promoting team work is essential to encourage a culture of clinical safety37,38 and its benefits can extend far beyond those observed in this experience. This field is not, however, free from complexity, and it would be a good idea to establish actions maintained over time to promote interdisciplinary collaboration and shared decision-making39 that include patients and their families as an integral part of the health team.
As with other nurse-led, multidisciplinary interventions,40 leadership and autonomy in decision-making was crucial in the intervention because, in addition to strict patient monitoring, it enabled frequent adjustments to the insulin therapy according to the clinical situation or treatment.
With regard to limitations, we must highlight that the design, the methods of evidence implementation and the efficacy of the interventions were greatly influenced by the context in which they were to be implemented, which limits their generalisation. In this sense, some specific aspects of the protocol depend on the preferences and agreements of the health team in the care and treatment of patients, therefore its format might require changes or tweaks for adaptation to other centres or units.
The sample size we obtained might be too limited to generalise the results. Therefore, the observation time was extended for 9 months after the intervention, although we cannot know the longer term impact of the recommendations included in the protocol or whether they were maintained after rotation of healthcare staff or possible changes to the unit's leadership structure.
Furthermore, as there was no previous information on the incidence of hyperglycaemia and hypoglycaemia in our centre, it was not possible to use an approach with methodologies that enabled comparison of the efficacy of the protocol with a baseline status. Similarly, some potentially confusing variables, such as the use of glucose-lowering drugs, could not be included in the analysis of the homogeneity of the sample.
Our intervention had a positive impact on the efficacy of glycaemic control in ICU, which could benefit patient safety. Therefore, our study could help towards the design and improvement of action protocols for nurse-led control of hyperglycaemia in critically-ill patients and in reducing clinical variability.
ConclusionsBy implementing the nurse-led protocol we obtained better glycaemic rates within the target range in the post-intervention group. Furthermore, there were fewer severe hyperglycaemias in this group, and no hypoglycaemia below 40mg/dl was observed in any case.
Our study shows that a multidisciplinary intervention led by nurses can be safe and effective in the control of hyperglycaemia in critically-ill patients.
FinancingThis study received a research grant from the Official College of Nursing of the Balearic Islands (556/14). The financial entity did not intervene in any phase of the research study.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the healthcare professionals of the Intensive Care Unit of the Monaco Hospital and the Official College of Nursing of the Balearic Islands (COIBA) for their collaboration in the study.
Please cite this article as: Rodriguez-Calero M.A, Barceló Llodrá E, Cruces Cuberos M, Blanco-Mavillard I, Pérez Axartell M.A. Efectividad de un protocolo basado en la evidencia para el control de lahiperglucemia por estrés en cuidados intensivos. Enferm Intensiva. 2019;30:4–12.