“One’s ideas must be as broad as nature if they are to interpret nature”.
Sherlock Holmes to Dr. Watson.
A study in Scarlet, Arthur Conan Doyle.
Patient-reported outcome measures (PROM) and patient-reported experience measures (PREM), offer us the patient-oriented assessment on aspects such as the effectiveness of an intervention or the safety of healthcare.1,2 These measures are broadly used in research and in quality optimisation studies and they are assessed through tools or questionnaires. For this the acronyms PROM and PREM are used directly to refer to the tool or questionnaire of measurement. Both types of measures have acquired extensive importance due to the current paradigm which places patients at the centre of healthcare and research.3,4
The purpose of this article is to describe the use and importance of the PROM and PREM for nursing practice and research, and also to disseminate the guidelines and tools which help to identify and select those which are most appropriate for a specific purpose, context and population group.
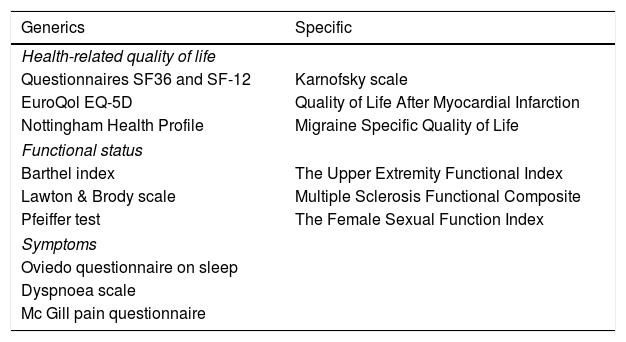
What do the PROM and PREM measure?The PROM are tools for measuring patient perceptions on their health status, their symptoms, their degree of autonomy or capacities, their well-being or health-related quality of life.2 There are 2 types of PROM: the specific ones, aimed at patients with a certain disease or condition, and the generic ones, applicable to any population group. In Table 1 there is a description of several frequently used PROM. In contrast, the PREM contain information from the patient’s viewpoint about the care received, preferably focusing on aspects relating to the humanity of care,2 which could include, among other aspects, empathy, dignity, respect, comprehension or personalized care.
List of some of the most used patient-reported outcome measures (PROM) s.
| Generics | Specific |
|---|---|
| Health-related quality of life | |
| Questionnaires SF36 and SF-12 | Karnofsky scale |
| EuroQol EQ-5D | Quality of Life After Myocardial Infarction |
| Nottingham Health Profile | Migraine Specific Quality of Life |
| Functional status | |
| Barthel index | The Upper Extremity Functional Index |
| Lawton & Brody scale | Multiple Sclerosis Functional Composite |
| Pfeiffer test | The Female Sexual Function Index |
| Symptoms | |
| Oviedo questionnaire on sleep | |
| Dyspnoea scale | |
| Mc Gill pain questionnaire | |
Both the PROM and the PREM represent standardised measures to quantify patient perspective and they help us to understand how disease, the health system and healthcare, impact patients.5 They are appropriate for patient follow-up and for being able to take informed and shared decisions on care and treatment. They are also useful as markers to compare different services or health providers from the viewpoint of quality optimisation. Similarly, in research, they facilitate the determination of intervention efficacy and also intervention effectiveness and cost-effectiveness. They are therefore helpful for identifying new ways of providing healthcare and planning equitable healthcare services. Lastly, we should not forget that patient-centred care is the essence of the nursing profession,6 and therefore both PROM and PREM are essential measures to offer quality care and assess its effectiveness and the impact of nursing interventions.
How do we identify and select the most appropriate tools of measurement?For these measures to adequately fulfil their function sensitive, valid and reliable tools of measurement are required which are also, preferably, fast and easy to apply. The appropriateness of a tool of measurement is assessed according to its ability to provide reliable and valid measurement in a certain population group, in a certain context and for a specific proposal. For example, a PROM or a PREM may be valid and reliable for carrying out a measure within the context of hospital practice, but not for community practice. Also, a valid tool for carrying out a screening may not be as valid for predicting events. For this reason the population, context and purpose are key elements for designing or selecting a tool. Equally, ethical aspects have been considered on deciding the use of one or more measurement tools. We must not forget that if information we collect with the tool is not valid or reliable it may lead us to the wrong conclusions which harm the patient, our practice and our research studies. Furthermore, the administration of this type of instrument means there is often an overburden for both the patients and for the professionals involved in their administration, and for this reason we must be highly certain that the effort involved will prove useful.
Notwithstanding, identifying and selecting the best measurement tool is a complex task. To do so, it is often the case that a new tool is created when it is thought or believed that none is available and often this is not true. In most cases the reality is that we were unable to find it. It is also true that many of the measuring tools have been validated for population groups of contexts which are different from those that interest us. The most efficient decision is always to adapt an available tool rather than create a new one.
Different existing resources facilitate both the identification of the measurement tools and the decision regarding their appropriateness, among which the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) initiative stands out. Its purpose is to develop practical methodologies and tools to identify and select the most appropriate measurement tools both for practice and research.7 Their web platform informs us from how to decide what to measure to how to carry out a systematic review of the measurement instruments (Fig. 1). Among all the tools provided by COSMIN we must highlight 2 for their particular usefulness in research.
Extract from the page of the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN), where the different available tools are described.
Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Available in: https://www.cosmin.nl.
The first is the compendium of guidelines for carrying out systematic reviews on measurement tools8 (Fig. 2). This type of review facilitates the selection of the most appropriate PROM and PREM for a certain purpose since it offers an exhaustive description of the psychometric properties of the measurement tools accompanied by usage recommendations based on the available evidence. The number of these reviews has increased in recent years and at present almost a hundred per year are indexed in bibliographic data bases such MEDLINE. In these reviews the characteristics of the PROM and PREM are assessed and summarised based on the methodological quality of validation studies which are carried out and the degree of quality criteria compliance of each one of their psychometric properties. The interpretability of the tool outcomes, and their feasibility to be administered, are also assessed. These reviews, like all systematic reviews, are highly useful for identifying aspects of a certain measurement tool which require researching, since the available tests are insufficient. The first step therefore to identifying the appropriate PROM or PREM is to look for systematic reviews of measurement tools on the construct or aspect we wish to measure.
Section from the COSMIN website dedicated to guidelines for carrying out systematic reviews of measurement tools.
Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. I’m conducting a systematic review of outcome measurement instruments. Available in: https://www.cosmin.nl/finding-right-tool/conducting-systematic-review-outcome-measurement-instruments/.
The second relevant tool is a validated bibliographic search filter which allows us to recover validation studies of measurement tools in bibliographic databases such as PubMed9,10 (Fig. 3). Finding studies on measurement tools is difficult, since they are not indexed homogeneously and there is a wide variability of terminology. This filter is very useful since adding it to search terms related to the construct we wish to measure, with the target population and the context of interest allows us to construct a highly sensitive and efficient search strategy (97.4% sensitivity and 4.4% precision). This filter has been adapted to databases such as EMBASE, PsycInfo and CINAHL, but has only been validated for PubMed.
Section from the COSMIN website dedicated to bibliographic search filters available for efficiently recovering studies on measurement tool validation.
Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Search filters. Available in: https://www.cosmin.nl/tools/pubmed-search-filters/.
In addition to COSMIN, there are other resources which could be highly useful to identify which PROM and PREM we can measure, and which available tools exist in particular when we wish to carry out experimental studies. In this sense the database Core Outcome Measures in Effectiveness Trials (COMET) is of note. These index the core outcome set (COS).11 The COS is a consensual set of standardised outcomes which have to be measured and reported in experimental studies of certain health areas or healthcare. Its database allows search to be made by study area, type of population, research methodology and other study characteristics which we wish to carry out, recuperating studies which are related and helping us to identify which are the most appropriate COS for our study.
To conclude, the phrase by Sherlock Holmes at the beginning of this article refers to the fact that the person forms part of the disciplinary nucleus of nursing, of their “nature”, and both the PROM and the PREM represent extremely useful tools if we “aspire to interpret them”. Knowing whether available measurement tools exist for our proposal and determining which is the most appropriate are essential processes for obtaining reliable and valid outcomes. Different tools exist that may help us in this identification and selection in a thorough manner based on scientific evidence.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Zabaleta-del-Olmo E, González-del-Río M. Instrumentos de medida de resultados y de experiencias comunicadas por el paciente: directrices y herramientas para identificar y seleccionar los más adecuados. Enferm Intensiva. 2020. https://doi.org/10.1016/j.enfi.2020.08.001









![Extract from the page of the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN), where the different available tools are described. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Available in: https://www.cosmin.nl. Extract from the page of the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN), where the different available tools are described. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Available in: https://www.cosmin.nl.](https://static.elsevier.es/multimedia/25299840/0000003200000001/v1_202102250952/S2529984021000057/v1_202102250952/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Section from the COSMIN website dedicated to guidelines for carrying out systematic reviews of measurement tools. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. I’m conducting a systematic review of outcome measurement instruments. Available in: https://www.cosmin.nl/finding-right-tool/conducting-systematic-review-outcome-measurement-instruments/. Section from the COSMIN website dedicated to guidelines for carrying out systematic reviews of measurement tools. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. I’m conducting a systematic review of outcome measurement instruments. Available in: https://www.cosmin.nl/finding-right-tool/conducting-systematic-review-outcome-measurement-instruments/.](https://static.elsevier.es/multimedia/25299840/0000003200000001/v1_202102250952/S2529984021000057/v1_202102250952/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Section from the COSMIN website dedicated to bibliographic search filters available for efficiently recovering studies on measurement tool validation. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Search filters. Available in: https://www.cosmin.nl/tools/pubmed-search-filters/. Section from the COSMIN website dedicated to bibliographic search filters available for efficiently recovering studies on measurement tool validation. Source: COSMIN [Internet]. Amsterdam: Dept. of Epidemiology and Biostatistics; 2020 [consulted 16 Sep 2020]. Search filters. Available in: https://www.cosmin.nl/tools/pubmed-search-filters/.](https://static.elsevier.es/multimedia/25299840/0000003200000001/v1_202102250952/S2529984021000057/v1_202102250952/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

