In the last two decades, non-invasive mechanical ventilation (NIV) has been consolidated as an initial strategy for the management of respiratory failure in critical adult and paediatric patients.
ObjectivesTo identify risk factors and preventive strategies to reduce the incidence of skin lesions associated with clinical devices (LESADIC) related to NIV, as well as the most effective treatment for injuries that cannot be avoided.
MethodologyReview in the MEDLINE, CINAHL and Cochrane databases of studies published in the last 10 years to reach consensus through an expert panel.
ResultsKnowledge about how to measure correct mask size and protection of the skin with foam or hydrocolloids dressings are factors related to the incidence of LESADIC, as it conditions the degree of pressure-friction and shear that the interface exerts on the skin. The interface that causes fewer LESADIC and is better tolerated is the face mask. When there are injuries, the first thing is to remove the interface that causes pressure on damaged skin, recommending a Helmet® hood as an alternative, treating the infection, managing the exudate and stimulating perilesional skin.
ConclusionsThe mask of choice is the facial, always using foam or hydrocolloid dressings on the nasal bridge. Evaluate the condition of the skin under the interface and harness every 4h (recommended) and 11h (maximum). Evaluate the rotation strategy of the interface at 24h if the NIV is still needed on an ongoing basis.
En las 2 últimas décadas la ventilación mecánica no invasiva (VNI) se ha consolidado como estrategia inicial para el abordaje de la insuficiencia respiratoria en el paciente crítico adulto y pediátrico.
ObjetivosIdentificar los factores de riesgo y estrategias preventivas para disminuir la incidencia de lesiones cutáneas asociadas a dispositivos clínicos (LESADIC) relacionadas con la VNI, así como el tratamiento más eficaz para las lesiones que no se han podido evitar.
MetodologíaRevisión en las bases de datos MEDLINE, CINAHL y Cochrane de estudios publicados en los últimos 10años para llegar al consenso mediante panel de expertos.
ResultadosLos conocimientos acerca de cómo medir la talla correcta de máscara y la protección de la piel con apósitos de espuma o hidrocoloides son factores relacionados con la incidencia de LESADIC, pues condiciona el grado de presión-fricción y cizalla que la interfaz ejerce sobre la piel. La interfaz que menos LESADIC causa y es mejor tolerada es la máscara facial. Cuando hay lesiones, lo primero es retirar la interfaz que provoca presión sobre la piel dañada, recomendando como alternativa el casco Helmet®, tratar la infección, gestionar el exudado y estimular piel perilesional.
ConclusionesLa máscara de elección es la facial, siempre con uso de apósitos de espuma o hidrocoloides en el puente nasal. Evaluar el estado de la piel debajo la interfaz y arnés entre las 4h (recomendable) y 11h (máximo). Valorar la estrategia de rotación de la interfase a las 24h si la VNI sigue siendo necesaria de forma continuada.
Over the last two decades the use of non-invasive mechanical ventilation (NIV) has tripled,1 becoming the first strategy for handling respiratory failure both in adults and in paediatric patients.2–4 However, the use of NIV is still associated with skin damage, connected to the medical equipment used during treatment.5–8 A recent literature review of 62 clinical trials6 showed a incidence rate of skin damage between 2% and 50%; however this increased to 100% after 48h on NIV. These changes in the incidence rate could be explained by the fact that only 45% of the studies in the review contained data regarding the incidence rate of complications, skin damage among other things. Previously, a review by Gay5 found an incidence rate of between 10 and 120%. Single centre studies9 have found rates of 7% in patients on NIV treatment for a period of >72h or 1.7% with majority use of facial masks, without specifying the among of time in contact therewith.10
Damage caused by the use of interfaces and/or harnesses are classified in the group of damage associated with medical equipment (MDRI), used for the purpose of diagnosing or treating, where the damage has the same form as the equipment.
In adults, this makes up for a third of all pressure ulcers11 and those associated with NIV make up for between 10% and 17% of all MDRI.12,13 With critically ill paediatric patients, pressure ulcers related to respiratory equipment have a prevalence rate of 60.1%, and 78.8% of these are specifically associated with continuous positive airway pressure (CPAP)14continuous positive airway pressure (CPAP).14
Handling the interface, the selection, attachment and fitting have an effect on the success or failure of the NIV, as well as the MDRI incidence rate.9,15,16 Furthermore, it has an effect on the patient's level of comfort, which is an often a reason for suspending the NIV and level of leakages, which is associated with patient-ventilator synchronisation, a risk factor that may cause the treatment to fail in patient with acute chronic respiratory failure.6 Cabrini et al.17 detected that in 42% of patients hospitalised, NIV failed due to incorrect handling of the interface and the nursing staff is mainly responsible since they are at the patients’ bedside 24h a day, monitoring the development of the treatment.
The aim of this document is to carry out a synthesis based on the scientific evidence regarding the risk factors and preventative strategies to reduce the effect of MDRI associated with NIV, as well as to draw conclusions on the most effective treatment for inevitable damage.
Justification for the need to draw up the documentGiven that there is still no ideal interface (minimum leakages, no damage buy stable, non-deformable, no allergens, with minimum dead space, low cost and available in various sizes); there also is no perfect fastening system for the correct attachment and maintenance (easy to attach and detach, breathable, compatible with various interfaces, reusable)15; and that there is no agreement as to what the best barrier would be to protect the skin,18,19 it is necessary to identify strategies to prevent skin damage associated with the interface and the harness.
Similarly, and in line with the foregoing, it is necessary for the scientific communities working with critically ill patients, regardless of the unit where they have been hospitalised (for example, intensive care or semi-critically ill, emergency services, post-surgery recovery, out-patient care, among others), with the help of the community of experts in the prevention of bedsore, to agree on basic skin-care recommendations for patients being treated with NIV.
Main and secondary objectives- (1)
To identify the mechanisms connected with the appearance of skin damage when on NIV in adult and paediatric patients.
- (a)
To discover the risk factors for the development of skin damage associated with pressure and friction caused by the interface.
- (b)
To identify other possible elements apart from the interface that might cause skin damage when on NIV (e.g. the harness).
- (a)
- (2)
To agree on measures to prevent skin damage associated with NIV in adult and paediatric patients.
- (3)
To collate recommendations for skin protection against pressure and friction from the NIV equipment.
- (4)
To identify the best treatment for skin damage associated with NIV in adult and paediatric patients.
Professionals who treat critically ill patients: nurses, doctors (ICU, anaesthetists, pneumologists, A&E), respiratory physiotherapists.
Population group studiedHospitalised acute care patients (ICU, semicritical care, post-surgery recovery, A&E), or on pre-hospital emergency care.
In this document, we have not taken patients with in-home NIV treatment because their basal conditions are very different to those of critically ill patients who are in a state of shock and often experience changes to their metabolism that severely affect their ability to protect themselves from skin damage.
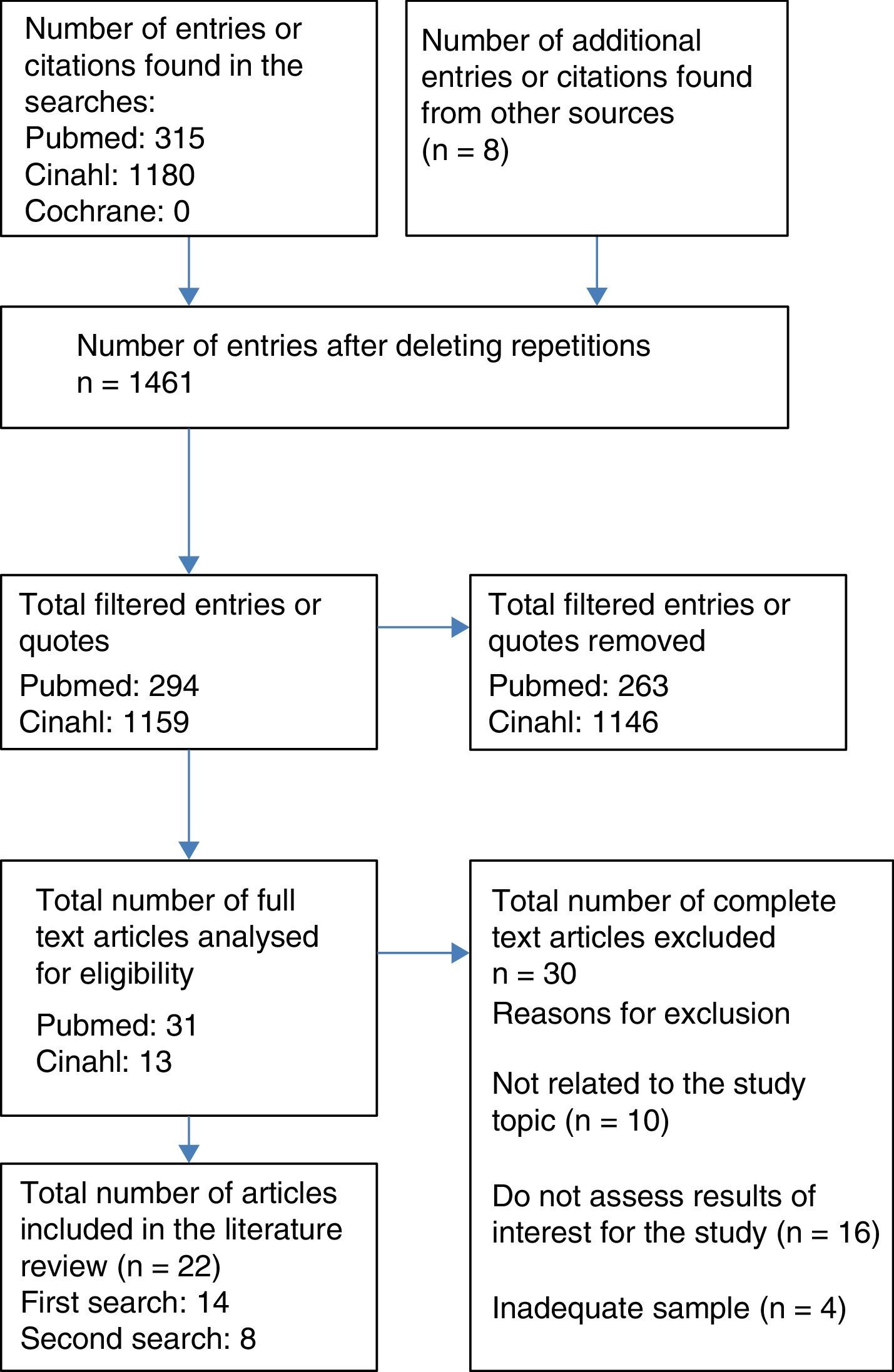
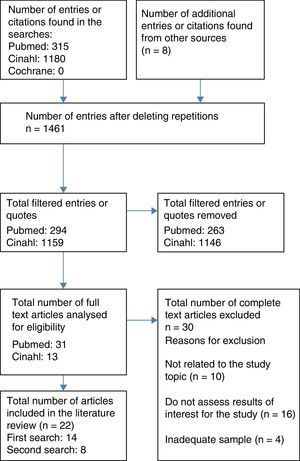
Resources usedA literature review was completed on 1 August 2016, by 4 independent reviewers for the study, through the MEDLINE database (via PubMed), CINAHL and The Cochrane Library. The process of which was to select articles with a flow chart for adult patients (Fig. 1). The articles were filtered down to those published in the last 10 years (2006–2016), with no limits on language, selecting those for adults aged 19 or over or paediatric patients from 0 to 18. The following terms were used to search for related scientific literature:
Pressure ulcers AND non-invasive ventilation.
Friction injuries OR moisture-associated skin damage OR skin lesions OR pressure ulcer AND mechanical ventilation.
Mask OR interface AND lesions AND non-invasive ventilation complications.
Friction injuries OR moisture-associated skin damage OR skin breakdown OR pressure ulcer AND mask OR interface.
Term MESH: masks/adverse effects.
Furthermore, a reverse search was conducted using the bibliographical references of the selected studies that, along with other sources that were not identified based on the review, are the articles cited as a secondary review in Fig. 1.
Type and number of bibliographical sources consultedThe total number of the articles selected was 30 and they were classified as follows:
- -
Risk factors identified (Table 1, additional material): skin condition, exposure time with the interface, number of NIV application and total time of each application, total hours on NIV, type of interface, type of ventilator/ventilator programme, team experience.
- -
Prevention strategy proposed (Table 1, additional material): use of dressings, use of inflatable cushions on the interface, interface rotation strategy, type of application of the interface, risk assessment scale and frequency of assessment, rest time between NIV applications.
- -
Treatment proposed for damage (Table 1, additional material).
- -
The same methodology was followed for the literature review on paediatric patients, which finally included 8 articles, the design and content of which are described in Table 3 (additional material), classified in the same was as with adult patients.
Out of the 30 studies, 3 had a level of evidence at Ib (randomised clinical trial), 2 at level IIa (non-randomised clinical trial) and 16 at level III or IV (non-experimental studies and/or panel of experts). Out of the remaining ones 5 were clinical case studies, 3 were literature reviews and one was a clinical handbook: the use of NIV in the management of patients with chronic obstructive pulmonary disease,20 in aspects regarding skin damage, there are only C-grade recommendations.
Methodology used to create recommendationGiven the differences in the level of evidence, the GRADE guide will not be used, but rather the recommendations based on the consensus of a panel of experts, subsequently reviews by scientific committee and/or work groups from critically-ill patient care societies (SEEIUC, SECIP) of the Grupo Nacional para el Estudio y Asesoramiento en Úlceras por Presión y Heridas Crónicas (‘The Spanish Pressure Ulcer Advisory Panel, GNEAUPP in its initials in Spanish) and the Asociación Internacional de Ventilación No Invasiva (‘International NIV Association).
Working methodologyThe literature sure was carried out independently by the reviewers in the study, sharing the documents selected online and communicating most often by email and video conference.
Document contentsDefinition and epidemiology of skin damage associated with non-invasive mechanical ventilationThe definition of pressure ulcers has developed over the years, and the most recent of these is the one from the GNEAUPP (2014)21,22: “Damage to the skin and/or adjacent tissue, generally over a bone protrusion, as a result of pressure or pressure combined with force of friction. It can also sometime appear in soft tissues that undergo external pressure from various materials or medical equipment.” Pressure ulcers are classified taking into consideration the depth of the affected tissue, and the GNEAUPP21 stipulates 5 stages and the National Pressure Ulcer Advisory Panel (NPUAP) and the European Pressure Ulcer Advisory Panel (EPUAP)23 6, since they add the “Unstageable pressure injur” to the 5 previous ones (Table 1).
Bedsore classification.
| Category | Definition |
|---|---|
| Stage I: Non-blanchable erythema | Skin intact with non-blanchable redness (capillary refill) in a localised area, generally over a bone protrusion, although it can also happen to soft tissues that undergo external pressure from various materials or medical equipment. |
| Stage II: Partial-thickness ulcer | Partial loss of thickness in the dermis, which shows as an open ulcer, without much depth, with a wound base that is usually pink or red and without slough. |
| Stage III: Full-thickness skin loss | Total loss of dermal tissue. Subcutaneous fat may be visible, but not deeper structures. The depth of this category may change according to the location on the body and the presence of subcutaneous adipose tissue, since the area where this does not exist are not very deep (bridge of the nose) or very deep where it does exist. |
| Stage IV: Full-thickness skin and tissue loss | Full-thickness tissue loss, with exposed bones, tendons or muscles. The depth varies according to the area of the body and the subcutaneous adipose that it has. |
| Deep tissue damage | Localised area with a more irregular form, (generally not as round as other pressure wounds), which normally shows a double erythema, the second being darker (purple or brown) and in the first, which may be 30°–45° away from bone crests. |
| Unclassifiable: depth unknown (included in the NPUAP classification system) | Complete loss of dermal tissue with the presence of slough and/or eschar in the base of the ulcer; until debridement, the true depth will not be known and it cannot be classified. When there is slough or dead tissue, we are dealing with a category III or IV ulcer. |
The patients with medical equipment have at 2.4 times more risk of developing bedsore than patients that do not use such equipment.24 As result of this, people who use a NIV are at risk of suffering from MDRI, since the main forces that cause such damage are pressure and friction. Alongside Moisture-Associate Skin Damage (MASD), “a lesion on the skin which appears as an inflammation and/or the erosion of the skin, caused by prolonged exposure to various sources of moisture which can cause skin irritation,” which are included in the new paradigm of dependence-related damage.21,22,25
MDRI is classified according to the type of medical equipment that caused them26:
- -
Respiratory devices: endotracheal tube, oronasal mask, oxygen therapy glasses, tracheostomy cannula.
- -
Venous lines: central venous line, peripheral venous catheter, for kidney dialysis, arterial lines, for measuring intracranial pressure, epidural.
- -
Devices in the gastrointestinal tract/genitourinary system: nasogastric/orogastric probes, enterostomy tube, urinary catheter, stool collection system.
- -
Monitoring devices: arterial pressure fittings, ECG cables, pulse oximentry.
- -
Risk prevention devices: antithrombotic therapy devices, mechanical fixings.
MDRI has a prevalence rate of 8.8–40%.11,13,24,26,27 An additional problem with MDRI is that it is detected when it is already in advanced stages. 74% is detected at stage III or IV, while ulcers at this stage that are not associated with medical equipment have a rate of 54%.24 Only 5% of MDRI is detected at stage I compared to 20% in damage that is not associated with medical equipment.13
70% of MDRI appears on the front of the neck, the chin, the occipital region or nose, and only 8% of damage not associated with medical equipment appears in these regions.24 Among the MDRI, the main ones are on the tip of the nose and these are the most severe (category II or III). According to Diez et al.,28 the incidence rate for damage on the nose is 5–20%. This is followed by damage on the cheeks, forehead and chin, but this is mainly erythema or category I. This can affect the mucous membrane, which means the conditions thereof should be regularly checked, as with the skin if in contact with medical equipment.26 The most common damage in paediatric patients is also on the bridge of the nose (39%), followed by the left cheek (30%), the right (18%) and the chin (3%).8,29
Physiopathology of skin damage associated with non-invasive mechanical ventilationWith MDRI, the damage is caused by the pressure that the device puts on the skin and the mucous membrane of the patient, causing tissue ischaemia and compromising the supply of oxygen and nutrients to the capillaries, as well as the removal of metabolic waste, which build up and can cause local vasodilation. This contributes towards the formation of an oedema and, therefore, pressure increases on the blood vessels, generating even more ischaemia and oedema. Oedema itself leads to the risk of damage because it strains the skin and makes it more fragile. Ulcers develop because of pressure applied to the skin and the tissue, exacerbated by the ischaemia-reperfusion cycles, with the formation of free radical toxins. Damage can be caused by an ischaemia cycle of just 2h.8
Furthermore, the medical equipment causes perspiration under the surface on which it rests, causing microclimate changes and can macerate the skin and increase the friction coefficient, which causes epidermis layers to move about and make the skin more fragile and susceptible to the effects of pressure.12
The other force that causes MDRI is friction, when two surfaces that touch move on the same axis but in opposite direction against teach other (the medical equipment and the skin).21,30 Friction can be one of two types: static or dynamic.22 In static fraction, when the device has been attached with too much pressure, there is a force against the patient's skin which, along with gravity, causes shear. In dynamic friction, it comes off from its initial position (because of sweat, moisture from condensation in the air in the equipment, the equipment being badly attached), along with resistance from the skin.
Special consideration for damage associated with non-invasive skin damage in critically ill patientsApart from the aforementioned effects of pressure, friction, shear and moisture, there are other risk factors for the development of skin damage associated with NIV,31,32 such as:
- -
Haemodynamic instability: increasing the inflammatory response locally, causing more oedema and tissue ischaemia. One indicator of this is average arterial pressure <60–70mmHg.32
- -
Vasoactive medication: causing vasoconstriction and, therefore, a drop in perfusion to the distal tissues, such as the skin.
- -
Nutritional deficiency: the lack of proteins changes oncotic pressure and leads to the formation of tissue oedema.
- -
During mechanical ventilation: this is associated with immobility, loss of conscience due to sedatives and pain killers, incontinence and the risk of moisture-associated damage. This is also related to haemodynamic and respiratory problems, causing deficient tissue oxygenation.
The amount of time spent on mechanical ventilation is been identified with a 10% higher risk of suffering from ulcers.33 According to Nijs et al.,34 the risk factors associated with the development of ulcers in critically ill patients are:
- •
In the first 24h after hospitalisation:
- -
Dopamine <5μg/kg/min (OR: 6.05; CI 95%: 1.88–19.54).
- -
Medical history of vascular disease (OR: 4.51; CI 95%: 1.99–10.24).
- -
Haemodialysis (continual or intermittent) (OR: 3.77; CI 95%: 1.03–13.86).
- -
- •
In the first 48h after hospitalisation:
- -
Medical history of vascular disease (OR: 2.85; CI 95%: 1.29–6.3).
- -
Haemodialysis (continual or intermittent) (OR: 9.43; CI 95%: 3.01–29.51).
- -
Mechanical ventilation (OR: 4.82; CI 95%: 1.74–13.36).
- -
Lastly, it is deemed that the risk of damage due to the basal condition of the skin can depend on factors such as old age, low levels of haemoglobin and albumin, a change in the leucocyte formula, vitamin D deficiency and the administration of corticoids, all of which are normal conditions for critically ill patients.13,35,36
Regarding critically ill paediatric patients, apart from the aforementioned risk factors, these others should be mentioned: low birth weight, gestation status and long time spent on the ICU14,18,37; also in children below the age of 2, as well as the risk of suffering from pressure ulcers.18
Preventative measures for skin damage associated with non-invasive mechanical interventionGeneral preventative measuresAccording to the NPUAP,23 anyone using medical equipment should be deemed as a patient at risk of developing a bedsore. It is recommendable to inspect the skin underneath the medical equipment at least two times a day to check for any possible damage, most frequently fluid contact or the presence of oedema.
Identifying the risk a patient may have of suffering a bedsore, through a scale approved for critically ill patients, both adults and paediatric patients (for example, the Braden scale), should be applied as soon as they are hospitalised and at regular intervals (or when any change happens) with all patients to classify their risk level.31,38
Rondinelli39 noted that critically ill patients with MDRI had low scores on the Braden subscale “Friction and Shear” had a 2.5 times higher risk of suffering from MDRI (CI 95%: 1.12–5.53).
Preventative measures for skin damage caused by pressure, friction and shearBefore NIV is used, it is necessary to apply dressings for moist wound healing, to mitigate the forces of pressure and friction.8,12,20,28,31,36,40–44 Weng35 showed that the use of moist wound healing dressings improved the damage incidence rate during NIV (53.3% vs 96.7%, p<0.001). Even though there is no evidence for one bandage being better that the rest, these diminish the effect of pressure and friction, preventing the mask from moving due to moisture or the effect of gravity, leading to optimum synchronisation between the patient and ventilator, without having to have the mask too tight on the skin.45 Furthermore, the dressings make it more comfortable for the patient.46
Other strategies recommended for reducing the pressure of the interface on the skin are: (1) allowing two fingers distance between the harness and the facial skin47; (2) hanging the circuitry of the ventilator to stop the mask moving,15 or (3) increasing the level of positive airway pressure (CPAP), which work with non-mechanical systems, such as Whisperflow.36 In mechanical modalities, the level of inspiratory positive airway pressure and expiratory positive airway pressure have not been connected to the incidence skin damage.41
In critically ill paediatric patients, it is important to choose the right size mask for the patient's face and to adjust the cords correctly to hold it on to face, using velcro.8,48
Preventative measure for controlling moistureThe NPUAP23 recommends keeping the skin dry and clean where it is under medical equipment. The application of a non-irritant barrier product cause help to keep the skin dry, since the microfilm formed on the skin can avoid the contact between external moisture and the skin.49 However, there are no studies about the different types of barriers for the skin that would allow for a consensus on the best option.19
It is recommended to use MWH dressings that do not gel to manage skin moisture, which macerates the skin and makes it more fragile.8,12,35
Preventative measures associated with the interface and/or ventilatorSelecting the interfaceCorrectly attaching the interface is of the utmost important for the NIV implementation to be successful. If it is not correctly supported, the interface can increase unintentional leakage and thereby patient-ventilator asynchrony, increasing autocycling and the inefficient respiratory efforts. However, if it is attached to firmly, it can increase the risk of skin damage, causing discomfort to the patient and low tolerance of the therapy, which if severe can lead to the NIV being removed.50,51 Therefore, it is essential to train health-care staff in the correct use of the interfaces (selecting the size and correct attachment).52 The Royal College of Physicians20 recommends using the measured that come with in the commercial packaging of some masks.
The mask chosen should be the facial one (covering the eyes, nose and mouth), because this leads to less damage,8,10,19,20,28,40,41 less pain on the bridge of the nose10,53 is better tolerated by patients40,54 despite causing claustrophobia in some.53
According to Raurell-Torredà et al.51 the face mask leaks less than the oronasal mask, so nurses fix it tighter onto the patients’ faces.
Damage associated with the face mask almost always appears on the bridge of the nose10 due to the problem of adapting to different nose shapes, we need more diverse sized interfaces or elastic material that would adapt to more prominent or curved noses. Therefore, it is recommendable to protect the bridge of the nose with bandaging (foam or hydrocolloids) to reduce the effect of pressure, friction or moisture due to the interface being in contact with this delicate zone.
The second choice for masks (to rotate the pressure points and avoid the negative effects on one mask in particular) it is recommended to use:
- •
Helmet® in:
- -
Patients without teeth or dentures that do not adapt to anatomical changes during their time in hospital or those with a low level of conscience, which means that dentures are not used for the patient's own protection.
- -
Patients that move their chin unconsciously (common in patients with Alzheimer's or Parkinson's, for example).
- -
Patients with a bear that cannot be shaved off because of the patient and/or their family's orders.
- -
Patients with skin damage on the bridge of the nose, the cheeks or chin.
The new version of Helmet36,47 should be used, which is attached with a rigid collar, not bands in the armpits. Regarding the previous model, the helmet has less dead space and works using a double circuit, reducing the risk of re-inhalation and allows for better patient-ventilator synchronisation with the support pressure mode or pressure assisted mode, always with a high PEEP.55
- -
- •
The oronasal mask (covering the mouth and nose):
- -
In patients without the problems described above.
- -
In patients that are prone to claustrophobia or are not collaborative and reject the face mask as a first option.
- -
Regarding the oronasal mask, care should be taken not to over inflate the cushions, because this detracts from the reason why they are used in some face mask models, to help adapt to the patient's face.36 Some authors argue for the use of interfaces with a forehead support to reduce the pressure on the bridge of the nose.28
Regarding paediatric patient, although the mask chosen should be the facial one because it causes less damage than the oronasal one and the nasal one, it should be pointed out that there is a nasal interface, “Sleep Weaver”, with an interface made of fabric,19 but there is little reported experience with this.
Attaching the interfaceAs with the areas where the mask touches the skin, when the harness is tightened on oedematous zones (with a higher risk of tissue ischaemia), this skin should be protected with dressings that distribute pressure and absorb humidity. The occipital region should be checked as often as the rest of the skin, since this is often forgotten with MDRI.56 With Helmet®, the areas of the skin in contact with the rigid collar should be protected with foam to reduce pressure.
Choosing a ventilatorUsing the Likert scale from 1 to 4, where 1 is a relaxed and comfortable patient and 4 is an irritated patient who cannot keep the mask one, at levels 3 or 4, uncollaborative patients, therapy should use specific NIVs, which are easier to handle and adapt quicker to changes in the leakage rate.57,58
In units that can use conventional ICU ventilators, the care team should be trained on the concept of tolerance to leakage before starting NIV, so that they tend not to overly tighten the mask against the skin, causing intolerance and the patient rejecting the treatment.51
Time exposed to the interfaceWhichever interface is chosen, the first check on skin and mucous membranes affected by the equipment (interface and harness) should be carried out in the first 2–4h form the start of NIV treatment, at the same time as the respiratory and blood gas check that need to be done to assess the effectiveness of the therapy.20 If the patients does not tolerate the removal of the interface, or if there are orders to not remove it,24 the multidisciplinary team should reassess the tracheal intubation and invasive mechanical ventilation instructions, because a delay in identifying the signs and symptoms of NIV failure has a greater mortality rate.1,59,60 If it is decided to continue with NIV, the condition of the skin and mucous membranes should be checked every 4h if the patient is tolerating with regarding respiration and blood gas.8,14,19,42,43,61,62
If the patients need continuous NIV, given that the maximum time for using the interface without a break is 11h,56 and specifically for patients that are unstable when the interface is being reattached, the mask should be removed and the skin and mucous membranes should be left to rest for at least 4h (recommendable) y 11h (risk). It is recommendable to remove the interface for at least 10min to ensure oxygenisaton,40 but 30min would be better, to check if the redness goes away or if it should be deemed a category Iulcer.56
Dressings do not prevent damage in patients with over 12h of continuous exposure to the interface, therefore the different interfaces should be alternated to rotate the pressure points, particularly if the interface is oronasal. If the chosen mask covers the whole face or is a Helmet®, the same one can be kept, while checking the skin and mucous membranes every 4h if possible, at most every 12h, observing the aforementioned rest periods.63
After the first 24h on NIV rotation of the interface should be assessed, because this considerably alters the risk of damage,41 this decision should be made on an individual basis, regarding the condition of the skin, how well the patient is tolerating the interface and the amount of time planned on NIV.
Treatment of skin damage associated with non-invasive mechanical ventilationThe literature review regarding the treatment of skin damage associated with NIV found two articles that presented a total of 3 case studies with different therapies42,64 and a clinical practice handbook that, among other things, set guidelines for the prevention and treatment of damage associated with medical equipment.23 (Table 2, additional material).
Classification-categorisation of damageWhen bedsore and other damage associated with NIV appear, it is recommendable to identify and classify them using the classification-categorisation system for lesions associated with dependency from the GNEAUPP,21 except for damage to the mucous membrane since this system cannot be used to categorise bedsore that happen to this tissue.
AssessmentAs part of an integrated assessment of the patient with skin damage, the assessment of the damage must be done once detected and it must be reassessed at least weekly, documenting such assessment. In these circumstances, and making the most of dressing changes, it is recommendable to check the bedsore or other damage, looking for signs that would show the need change the treatment (skin deterioration, setbacks in development, increase in exudate, signs of infections or other complication).
It is necessary to assess the physical features of the damage; of the utmost importance are: location, category, size, type of tissue(s), colour, skin around the wound, edges, exudate and smell. Other characteristics to be taken into consideration, especially with people with darker skin tones, are whether the skin is hot or sensitive, changes in the consistency of the tissue and if there is any pain.
Check-ups on the damageThe healing process for damage need to be correctly monitored using approved and trustworthy tools (PUSH, DESIGN-R or RESVECH 2.0 scales, for example), supported by the medical judgement of a professional expert.65
PainIt is specifically necessary to assess the presence of pain caused by the wound or the treatment, stipulating the right measures to correctly deal with this.
Treating the woundCaring for wounds associated with NIV, as with those with other causes, starts with preparing the base. involving the control both of non-viable tissue and the infection or inflammation and the exudate, as well as stimulating the epithelial edges, through various interventions:
CleaningThe ulcer and the skin around it should be cleaned every time the dressings are changed, using drinking water or medical saline solution. Some cleaning solutions could be considered for use if there is an infection or suspected infection. In any case, it is useful to apply the right amount of pressure when cleaning without damaging the tissue or getting bacteria into the wound.
DebridementIt is recommendable to debride the devitalised tissue in the pressure ulcers, using the right debridement method(s) according the patient's general condition, the base of the wound and the main care objectives. Debridement should only be carried out if there is correct perfusion in the wound and if the team has the right knowledge and skills to do this. It is important to consider all the advantages and disadvantages of the different debridement options in detail, given the common locations of the damage caused by NIV and the possible aesthetic consequences.
Managing infectionsIt is necessary to plan and manage the bacterial load/local infection in the lesion if there is friable granulation tissue, if there are no signs of healing over two weeks, a foul smell, increase in size, increase in pain, increase in erythema and/or heat around the wound, increase and/or change in the exudate and/or increase in dead tissue in the wound.
Use of dressings in treatmentThere is a wide range of dressings on the market, and it would be going beyond the scope of this document to describe them all. If there is no definite evidence for recommending dressings over different treatment for the damage caused by NIV, the therapy decision should be based on the professional's knowledge of the product, on the evidence available, both internally and externally, on the profile of the patient and on the specific stage that the wound is at in the healing process. The criteria to be assessed at the moment of selecting the dressing(s) included, the ability to keep the base of the wound sufficiently moist, the need to handing the bacterial charge, the nature and volume of the exudate, the condition of the tissue in the base of the ulcer, the condition of the skin around the ulcer, the size of the wound, the depth and location, the presence of tunnels and/or cavities and their compatibility with the medical equipment used.
General considerationsIt is important to note the treatment of this damage is complex since it had to be compatible with care and the continued use of the equipment that caused it. Therefore, alongside the treatment and local care for these wounds caused by pressure, friction and/or shear as a result of NIV, the different preventative measures mentioned above should be kept up (regarding the interface and ventilator, attachment system, pressure relief, etc.).
Similarly, measures to should be continued to implement, maintain and develop the different risk and skin assessment processes, to control pressure, shear and friction, to control moisture and the contributory factors, in order to prevent other damage associated with dependency.
ConclusionsThe authors of this document, with the support of the scientific community that backs them, recommend:
- •
The face mask as the interface of choice. The oronasal mask should only be chosen for claustrophobic patients. In patients with MDRI, Helmet® should be considered for use to avoid the effect of pressure and shear where the interface touches damaged skin when it is necessary to continue with NIV.
- •
Assessing the use of Helmet® in place of the face mask in patients that move their chin involuntarily.
- •
Protecting the skin on the bridge of the nose with polyurethane foam dressings with silicone adhesive (to reduce pressure and friction) or hydrocolloid dressings when there is only the risk of friction, as is the case with the face mask and the areas where the harness rubs.
- •
Identifying the risk of pressure ulcers with the Braden scale upon hospitalisation and at regular intervals (or if there is any change in the patient's general condition).
- •
Assessing the condition of the skin and mucous membrane after 2–4h from starting NIV, at the same time as the haemodynamic and respiratory assessment, to check that the therapy is effective.
- •
If NIV is to be continued, assessing the skin every 4h (recommended) and at least every 11h (risk).
- •
After 24h on NIV, assessing the interface rotation strategy, because the dressings stop protecting the patient from possible damage.
- •
Following the instructions given in clinical handbooks for cleaning, debridement, and the use of dressings in the treatment of MDRI.
The authors declare that they have no conflicts of interests.
Please cite this article as: Raurell-Torredà M, Romero-Collado A, Rodríguez-Palma M, Farrés-Tarafa M, Martí JD, Hurtado-Pardos B, et al. Prevención y tratamiento de las lesiones cutáneas asociadas a la ventilación mecánica no invasiva. Recomendaciones de expertos. Enferm Intensiva. 2017;28:31–41.