To identify the available information to support registered nurses’ clinical decisions in assessing and validating potential organ and tissue donors during the COVID-19 pandemic.
MethodThis is a scoping review developed in six stages. The sixth stage was developed with registered nurses who work in the Brazil Organ Donation System. To consolidate the information and prepare all assumptions, the legislation in force in Brazil was followed.
ResultsRecommendations from 19 articles identified in the literature were analyzed; additionally, 52 professionals who work at Brazil Organ Donation System participated in the research. Four care assumptions were formed: investigation of community transmission, investigation of clinical situations, screening for COVID-19 signs and symptoms, and investigation of alterations presented in the physical examination. Such assumptions are formed by 34 care guidelines.
DiscussionCare assumptions were prepared to guide and support registered nurses during assessment and validation of potential organ and tissue donors. From this perspective, assumptions certainly promote safety, effectiveness and quality in the service offered during the organ and tissue donation process in the midst of the COVID-19 pandemic, in addition to empowering registered nurses in this scenario. Quality and bio-surveillance through the donation stages have been discussed extensively in recent times, to improve donation and transplantations by valuing care, safety, and quality of life of recipients.
ConclusionThe care assumptions presented in this study support and subsidize the daily practice of registered nurses who work in assessing and validating potential organ and tissue donors, enabling these professionals to make decisions based on secure information.
Identificar la información disponible para respaldar las decisiones clínicas de las enfermeras tituladas en la evaluación y validación de los donantes potenciales de órganos y tejidos durante la pandemia de COVID-19.
MétodoSe trata de una revisión del alcance, desarrollada en seis etapas. La sexta etapa se efectuó con las enfermeras tituladas que trabajan en el Sistema de Donación de Órganos de Brasil. Para consolidar la información y preparar todos los supuestos, el estudio se adaptó a la legislación vigente en Brasil.
ResultadosSe analizaron las recomendaciones de 19 artículos identificados en la literatura; además, 52 profesionales que trabajan en el Sistema de Donación de Órganos de Brasil participaron en el estudio. Se formaron cuatro supuestos de cuidados: estudio de la transmisión comunitaria, estudio de las situaciones clínicas, cribado de los signos y síntomas de la COVID-19, y estudio de las alteraciones presentadas en el examen físico. Dichos supuestos están formados por 34 directrices sobre cuidados.
DiscusiónSe prepararon supuestos sobre cuidados para orientar y respaldar a las enfermeras tituladas durante la evaluación y validación de los donantes potenciales de órganos y tejidos. En esta perspectiva, los supuestos promovieron ciertamente la seguridad, efectividad y calidad del servicio ofrecido durante el proceso de donación de órganos y tejidos en medio de la pandemia de COVID-19, además de empoderar a este grupo de profesionales en este escenario. En los últimos tiempos, se ha debatido ampliamente sobre la calidad y bio-vigilancia a través de las etapas de la donación, a fin de mejorar esta y los trasplantes, valorando los cuidados, la seguridad y la calidad de vida de los receptores.
ConclusiónLos supuestos sobre cuidados presentados en este estudio respaldan y subsidian la práctica diaria de las enfermeras tituladas que trabajan en la evaluación y validación de los donantes potenciales de órganos y tejidos, lo cual permite la toma de decisiones por parte de este grupo de profesionales, basada en información segura.
Assessing and validating a potential organ and tissue donor is a complex procedure, and it is necessary that this action be carried out strictly, especially in relation to physical and clinical examinations. Besides that, brain-dead patients have numerous physiological changes that generate signs that resemble those shown by critically ill patients with COVID-19.
What it contributes?The article presents an easy and quick guide for assessing and validating potential donors, addressing the main lines of investigation. In the current scenario, there is a need to look for all COVID-19 signs since all recommendations are for no transplantation from donors with COVID-19 to be performed. Care assumptions provide support for registered nurses to assess and validate potential organ and tissue donors amin the midst of the COVID-19 pandemic.
Implications of the studyThis study supports nurses’ decision-making on how to proceed to assess and validate a potential organ donor in the midst of the COVID 19 pandemic. With this research, we clarify the doubts of these professionals and guide the clinical investigation to guarantee a safe transplant process for all recipients. Therefore, it's an important study that have an immediate impact on the intensive nursing care practice of organ and tissue donation.
The COVID-19 pandemic has presented the world with a scenario of fear, doubts and uncertainties regarding its treatment, prevention, dissemination, and outcomes of contaminated individuals. Many advances have been made to understand the pathogenic potential of this virus; however, there are still many gaps. The scenario in healthcare has been one of the most affected by this pandemic; healthcare professionals face fears and concerns when making decisions regarding continuity of treatments, procedures and interventions for patients who need treatment for chronic pathologies.1
The scenario of concern, doubts, fear and insecurity also affects registered nurses working in Brazil Organ Donation System, as they are faced with the need to fulfill their role in identifying, assessing and validating potential organ and tissue donors. This occurs due to the chronicity and severity of patients who are on the waiting list for an organ transplantation. Prior to the pandemic, this was already an activity of great challenge imposed on registered nurses. Facing this new reality, the activity has started to be carried out with concerns about the criteria to be taken into consideration to validate a patient who is in a critical care unit as a potential donor, after the diagnosis of brain death is concluded. In this new scenario imposed by the pandemic, taking responsibility for validating a potential donor is a challenge that involves the need for knowledge based on evidence and effective strategies that can support decision-making.2
Assessment and validation of potential organ and tissue donors for transplantation purposes must take into account the information of all professionals who assisted/cared for this patient during their hospitalization. Every detail is important, considering that a potential donor can benefit several people through donation. Thus, if any fact or risk of contamination is not identified, several recipients may be harmed and adverse events may occur, causing health issues or even death.3–5
In the COVID-19 pandemic scenario, organ transplantation staff face several challenges, such as the likelihood of cross-infection among staff members, as well as asymptomatic and undiagnosed individuals with the COVID-19 virus who may act as contamination vectors between donors, recipients and health staff. Other uncertainties are related to the signs and symptoms that indicate COVID-19 presence, either due to the fact that patients are asymptomatic, and also because brain death happens in acute situations and in relatively young or middle-aged patients. Thus, assessing and validating potential donors has certainly been a delicate and stressful time for registered nurses in Brazil Organ Donation System and for the State Transplant Center's technical staff.6,7
Brazil, as well as several other countries, issued a technical note in which there are recommendations to be adopted regarding possible donors with confirmed COVID-19; clinical suspicion or cure; and those with clinical suspicion and no contact with suspected or confirmed cases. Furthermore, in the guidelines issued by other countries, there are strong recommendations to screen all possible donors using the RNA test for the virus using a nasal and pharyngeal swab. It is noteworthy that some countries have created multidisciplinary staff to discuss each case.6,8–11
It is believed, therefore, that consolidating the information will enable the development of care assumptions for best practices regarding clinical history, physical examination, epidemiological screening, and checking of tests to be performed on potential donors. Care assumptions support best practices. According to the World Health Organization, best practices are formed by a triad of considerations about users’ needs: experience, research and reliability.12 Collegiate Board Resolution 63 from 2011 reinforces that best practices are anchored in the principles of qualification, humanization, management, reduction and control of risks to users and the environment.12,13 From the best-practice perspective, care assumptions to be developed will certainly support registered nurses in decision making, in addition to minimizing risk of contamination to recipients/users who will be transplanted after organ donation.
Considering the facts here in exposed, the guiding question of this study is: What information is needed to support registered nurses’ clinical decisions in assessing and validating potential organ and tissue donors during the COVID-19 pandemic? This study aims to identify the available information to support the registered nurses’ clinical decisions in assessing and validating potential organ and tissue donors during the COVID-19 pandemic.
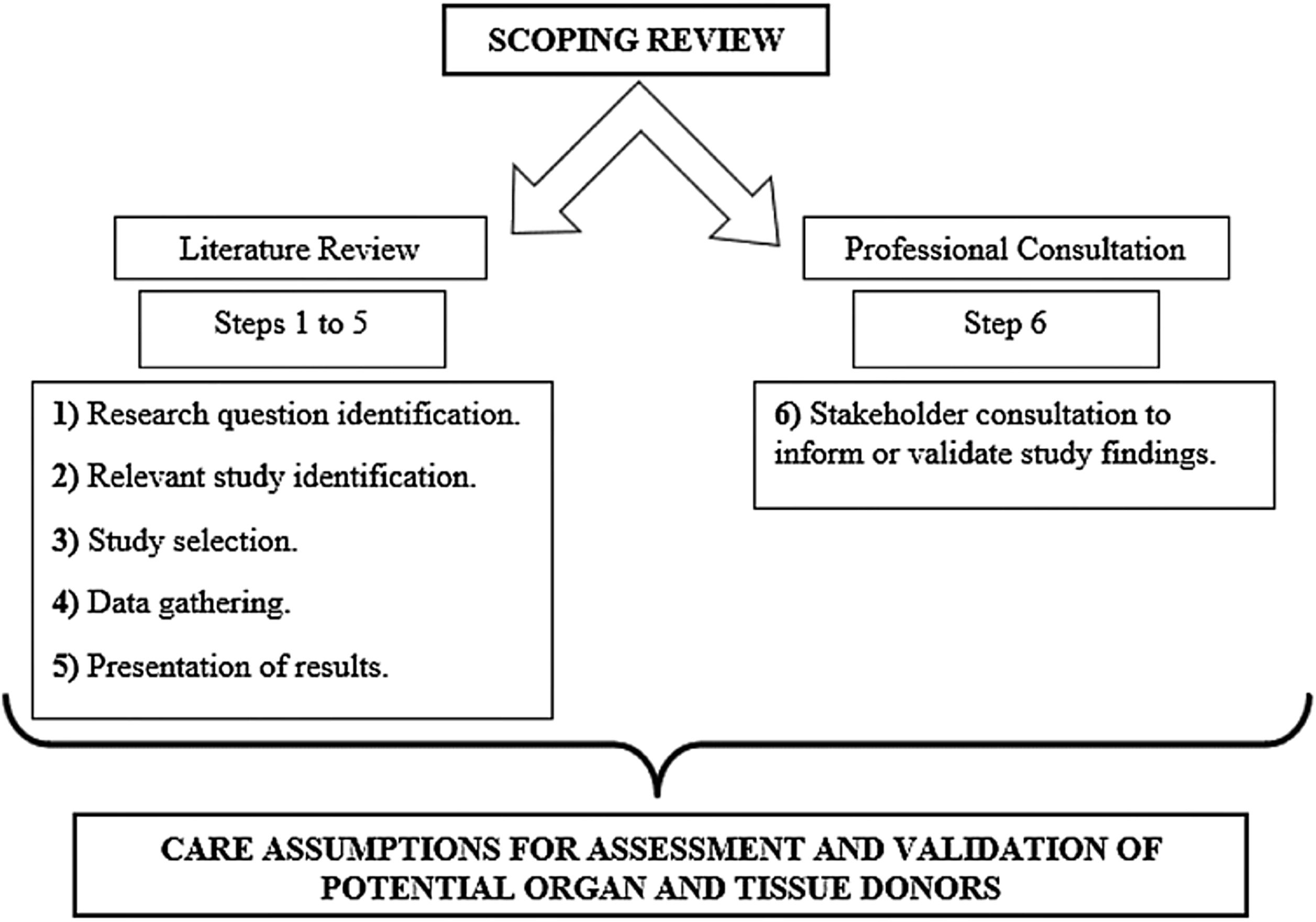
MethodsThe research method was developed as shown in Fig. 1. Below, the entire description of its process can be found.
Study method development.
Source: Theoretical framework16; authors, 2020.
This is a scoping review study developed using the method proposed by the Joana Briggs Institute Reviewers Manual and the Arksey and O’Malley theoretical framework; this study followed the following steps: (1) research question identification; (2) relevant study identification; (3) study selection; (4) data gathering; (5) presentation of results; (6) stakeholder consultation to inform or validate study findings.14
Data collection for literature review was carried out in the Medical Literature Analysis and Retrieval System Online (MEDLINE) and consulted through PubMed, Science Direct, SCOPUS and Web of Science.
The following descriptors were used: 2019-nCoV, COVID-19, coronavirus, SARS-CoV-2, transplantation, and donor organ assessment. It should be noted that search strategies were built in each database with descriptors, keywords and Boolean operators, such as ((COVID-19) AND transplantation); ((((coronavirus) AND COVID-19) AND 2019-nCoV) AND transplantation).
In order to deepen the discussion about the results found in the review, the first search conducted in May 2020 is available in preprint15; however, the results obtained in another search conducted in August 2020 are also presented in the current study.
The research was done by each author independently. First, all the authors found articles in the databases and reading titles and abstracts, then, the authors placed the articles in a bibliographic manager (Mendeley®) in order to exclude repeated studies. All authors read the articles complete and fed a spreadsheet in Excel® with the following data: title of the article, year of publication, author, country of publication, objective and main results. In case of disagreement between the authors, everyone read the article again until reaching a consensus.
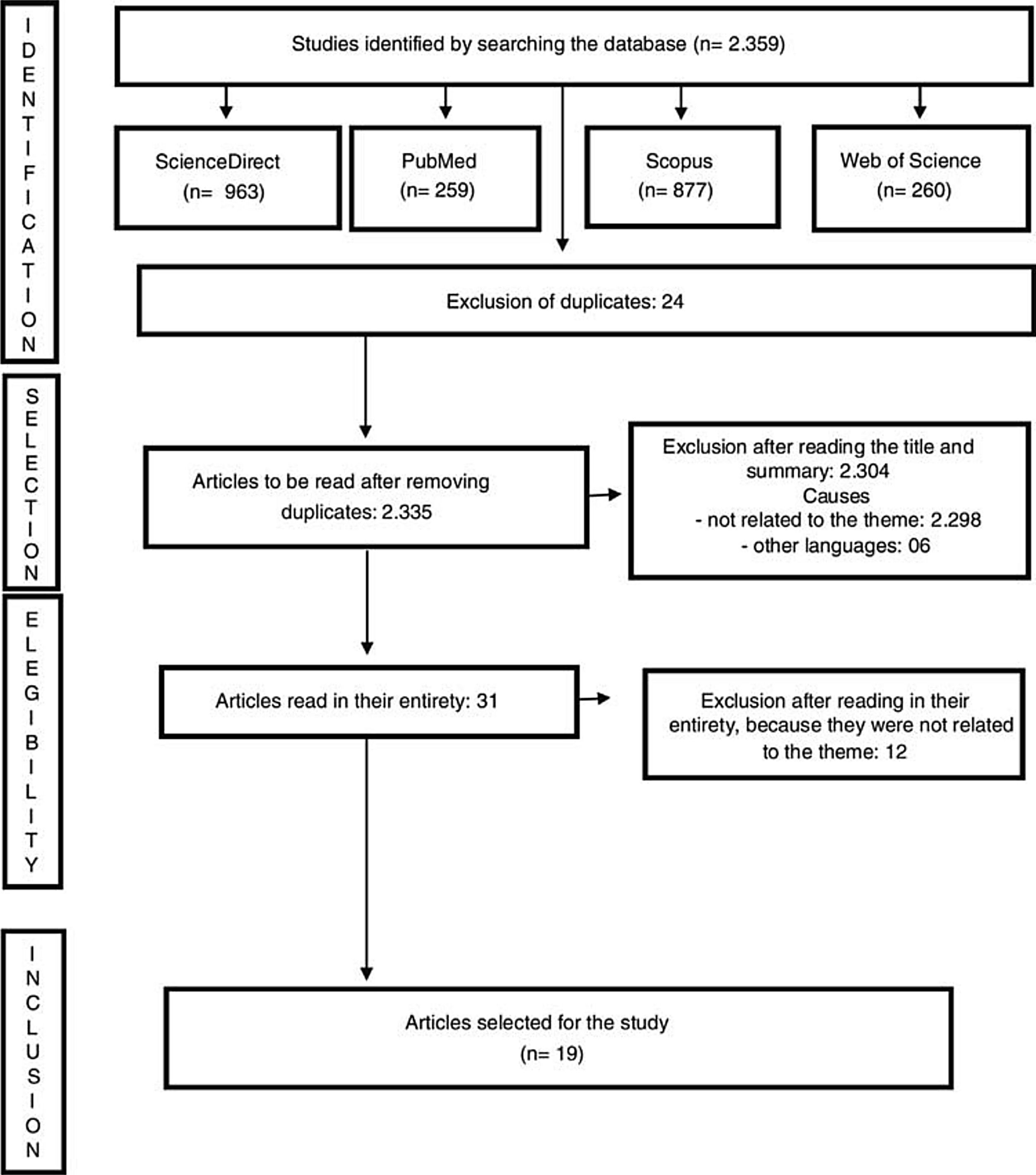
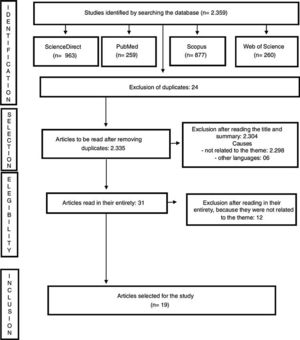
Inclusion criteria were articles with recommendations for clinical decisions for the evaluation and validation of potential organ and tissue donors during the COVID-19 pandemic, in Portuguese, Spanish and English, published between 2019 and 2020. Exclusion criteria were full texts unavailable even after communication with the authors of the work and articles with methodological problems identified by the authors. The review diagram is demonstrated in Fig. 2.
Review diagram.
Source: Joanna Briggs Institute Scoping Review Protocol.14
Regarding consultation with professionals, data collection was carried out in May and June 2020 using a three-part questionnaire developed in Google Forms. This questionnaire was prepared by four of the authors with years of experience in organ and tissue donation and transplantation, based on a technical note written by a national health agency. The questions asked were to find out all the details of the development of organ and tissue donation during the COVID-19 pandemic. The first one was the Informed Consent Form; the second part used five questions related to participant socio-cultural characterization; the third part had 10 questions related to assessment and validation of potential organ and tissue donors.
Four of those questions sought to identify the staff's strategies for dealing with assessing and validating potential donors. The other six questions sought to identify which investigations participants have been carrying out regarding clinical suspicion, signs and symptoms, hospitalization history, clinical investigation through with relatives, investigation regarding community transmission, physical examination, laboratory tests and imaging tests. First, the form was validated by three colleagues who are experts in organ and tissue donation and, subsequently, it underwent adjustments to better understand all professionals who would participate in the research.
In order to proceed with data collection, one of the researchers initially contacted all State Transplant Centers’ coordinators by email, explaining the research purpose and forwarding a link to access the questionnaire at Google Forms. By occasion of research being carried out by the State Transplant Center, its coordinator forwarded an email to professionals working at the State Transplant Centers and at the Organ Procurement Organization. Participants, when starting to fill out the form, agreed to the Informed Consent Form online, and then they had access to filling out the questions. The deadline for filling in was 15 days. The letter P was used to identify participants, followed by the order of return of the completed forms (e.g. P1, P2, and so forth).
Inclusion criteria was registered nurses and professionals working directly in the technical field at State Transplant Centers and at the Organ Procurement Organization. Exclusion criteria was registered nurses who were on vacation or away, as well as those who were temporarily replacing a professional.
There was no sample calculation in the part of consultation with professionals, since it was expected to obtain the largest number possible of completed forms. However, data collection ended when the information on the forms was repeated.
The socio-cultural characterization data of participants were organized in an Excel spreadsheet and analyzed descriptively. The data related to assessment and validation of potential organ and tissue donors were also organized in an Excel spreadsheet and, later, the most relevant information was analyzed. Consideration was given to the assessment and validation norms about potential organ and tissue donors, as provided in current Brazilian legislation, and in the Guidelines of the Brazilian Intensive Care Association; World Health Organization triad for best practices; Principles present in the Collegiate Board Resolution 63 from 2011, and Technical Note 34/2020.3,4,8,12,13
This research complied with all norms involving ethical aspects in research involving human beings and it was approved by the Research Ethics Committee of Federal University of Santa Catarina under Opinion 4,049,851 and CAAE (Certificado de Apresentação para Apreciação Ética – Certificate of Presentation for Ethical Consideration) 31782620.5.0000.0121.
After the described steps, care assumptions were developed to support registered nurses in decision-making. In addition to the assembly of steps 1 to 6 of this scoping review, the assumptions are anchored by the legislation in force in the country and by the national guidelines for assessment and validation of potential organ and tissue donors. Four moments led to this development:
At first, all researchers read all the obtained information exhaustively (1 to 6 from the scoping review). It is worth mentioning that four of the study researchers have over 15 years of experience in the organ and tissue donation process, both in the assistance field and in the academic research field.
In the second moment, the information was consolidated by each researcher in isolation.
In the third moment, two online meetings were held to define and consolidate the information that would make up care assumptions.
In the fourth step, care assumptions were elaborated, considering: investigation of community transmission, investigation of clinical situations, screening for COVID-19 signs and symptoms, and investigation of alterations presented in physical examination. Such assumptions are anchored by 34 care actions that are capable of supporting registered nurses in decision-making for assessment and validation of potential organ and tissue donors.
ResultsNineteen articles related to the theme were identified. The main focus of recommendations to assess and validate potential organ and tissue donors is directed at the development of the SARS-CoV-2 detection test and chest tomography for all patients, even for asymptomatic patients.
An alert was identified regarding the investigation of the presence of such patients in endemic regions, contact with healthcare environments and healthcare staff, as a risk of possible contamination. The recommendations identified in the scientific literature provide theoretical support for creating the assumptions that have been developed in this research and that are available in Table 1.
Recommendations for best practices in obtaining tissues and organs for transplantation.
| Title | Recommendations for assessment and validation of potential organ and tissue donors |
|---|---|
| Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.18 | Perform chest tomography and pay attention to acute respiratory distress syndrome, fever, cough, myalgia or fatigue, sputum production, headache, dyspnea and lymphopenia. |
| Organ donation during the coronavirus pandemic: an evolving saga in uncharted waters.19 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test 24h before donation and exclude donation in positive cases for COVID-19 or even in suspect cases presenting a negative result. |
| Challenges and countermeasures for organ donation during the SARS-CoV-2 epidemic: the experience of Sichuan Provincial People's Hospital.12 | Watch for fever, dyspnea, dry cough and recent diarrhea. Perform laboratory test and chest tomography. Screen contact with patients with COVID-19, and staff should have no history of contact with these patients. The staff must have been trained. |
| The COVID-19 outbreak in Italy: Initial implications for organ transplantation programs.20 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test 24h before donation. |
| COVID-19: A global transplant perspective on successfully navigating a pandemic.21 | Test potential donors and even asymptomatic patients. |
| Immediate impact of COVID-19 on transplant activity in the Netherlands.22 | Implement the SARS-CoV-2 test for all deceased donors and chest tomography scan, even for asymptomatic ones. |
| Dramatic impact of the COVID-19 outbreak on donation and transplantation activities in Spain.23 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test 24h before donation and wait 21 days after the donor was cured for donation to occur. Cancel the donation in case of positive COVID-19 cases. |
| Early impact of COVID-19 on transplant center practices and policies in the United States.24 | Test all donors, even low-risk ones, for COVID-19 using Reverse-Transcriptase Polymerase Chain Reaction Test. |
| Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases.25 | Pay attention to vesicular injuries, herpes zoster and skin injuries. |
| Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China.26 | Pay attention to loss of the sense of smell, fever, fatigue, gastrointestinal symptoms, signs of bilateral frosted glass or irregular opacity in radiological findings, lymphopenia and eosinopenia. |
| Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province.27 | Perform chest tomography and watch for fever, cough, muscle pain or fatigue and lymphocytopenia. |
| Clinical Characteristics of Coronavirus Disease 2019 in China.28 | Perform chest tomography and pay attention to lymphocytopenia, fever on admission and during hospitalization, and cough. |
| COVID-19: Yet another coronavirus challenge in transplantation.29 | Avoid organ transplantation from donors with a history of contact with someone at risk or diagnosed with COVID-19, or who have traveled to an area with a high density of infection. In suspect cases, even if the test is negative, avoid donation. |
| Management of an organ donation process in COVID-19 pandemic: first case of Turkey.30 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test, chest tomography and investigate family contacts and traveling history. |
| Strategies to halt 2019 novel coronavirus (SARS-CoV-2) spread for organ transplantation programs at the Sichuan Academy of Medical Science and Sichuan Provincial People's Hospital, China.31 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test, chest tomography and do not perform transplantation from donors who were in endemic areas. |
| Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic.32 | Investigate exposure history (traveling or presence in endemic locations); perform Reverse-Transcriptase Polymerase Chain Reaction Test and chest tomography; watch for cough, dyspnea and fever. |
| Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI).33 | Test potential donors for COVID-19, and donation can only occur if it is in the same city as the recipient. |
| Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A By-proxy SocietyRecommendation Consensus approach.34 | Perform Reverse-Transcriptase Polymerase Chain Reaction Test, watch for fever, cough, shortness of breath and gastrointestinal symptoms, and conduct research on community transmission. |
| Strategies for prevention and control of the 2019 novel coronavirus disease in the department of kidney transplantation.35 | Make donation unfeasible if potential donors have been at and/or traveled to endemic locations; watch for fever, dyspnea, dry cough and diarrhea; perform Reverse-Transcriptase Polymerase Chain Reaction Test and chest tomography. Donors may not have had companions during hospitalization. |
Source: literature review, 2020.
Concerning the last stage (6th), 52 professionals who work at State Transplantation Centers and Organ Search Organization of São Paulo participated in the survey; 82.7% of them are female; 57.7% are married; 51.9% are Catholic; 92.3% have a graduate degree; 90% are graduated and have been working for more than ten years with organ and tissue donation.
Of the questions related to assessment and validation of potential organ and tissue donors, 61.5% of participants pointed out that at their State Transplant Center, a differentiated routine was adopted that helps in screening for COVID-19 in potential organ donors. However, less than half, 48.1% said that this healthcare staff was trained/qualified to manage potential donors in this context of the pandemic. Only 30.8% stated that some epidemiological screening routine for professionals involved in organ donation was adopted.
Additionally, 56% of participants described other strategies, such as performing a chest tomography; chest X-ray for all donors; laboratory tests and rapid test for all potential donors in some State Transplant Centers, and, in others, carrying out Reverse-Transcriptase Polymerase Chain Reaction test (tracheal secretion) for all potential donors.
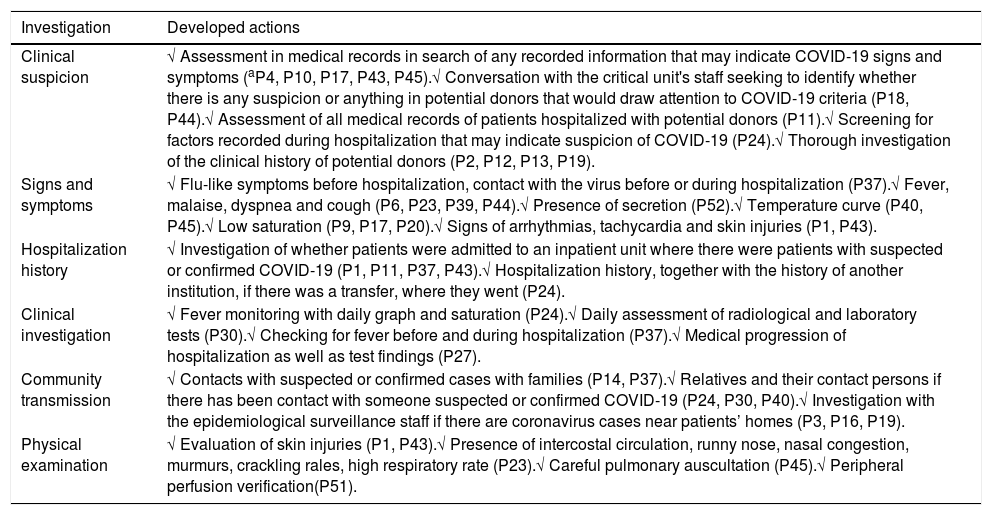
The information obtained through participants related to clinical suspicion, signs and symptoms, hospitalization history, clinical investigation, investigation regarding community transmission and physical examination, is available in Table 2.
Consolidation of information for assessment and validation of potential donors in the midst of the COVID-19 pandemic, according to professionals.
| Investigation | Developed actions |
|---|---|
| Clinical suspicion | √ Assessment in medical records in search of any recorded information that may indicate COVID-19 signs and symptoms (aP4, P10, P17, P43, P45).√ Conversation with the critical unit's staff seeking to identify whether there is any suspicion or anything in potential donors that would draw attention to COVID-19 criteria (P18, P44).√ Assessment of all medical records of patients hospitalized with potential donors (P11).√ Screening for factors recorded during hospitalization that may indicate suspicion of COVID-19 (P24).√ Thorough investigation of the clinical history of potential donors (P2, P12, P13, P19). |
| Signs and symptoms | √ Flu-like symptoms before hospitalization, contact with the virus before or during hospitalization (P37).√ Fever, malaise, dyspnea and cough (P6, P23, P39, P44).√ Presence of secretion (P52).√ Temperature curve (P40, P45).√ Low saturation (P9, P17, P20).√ Signs of arrhythmias, tachycardia and skin injuries (P1, P43). |
| Hospitalization history | √ Investigation of whether patients were admitted to an inpatient unit where there were patients with suspected or confirmed COVID-19 (P1, P11, P37, P43).√ Hospitalization history, together with the history of another institution, if there was a transfer, where they went (P24). |
| Clinical investigation | √ Fever monitoring with daily graph and saturation (P24).√ Daily assessment of radiological and laboratory tests (P30).√ Checking for fever before and during hospitalization (P37).√ Medical progression of hospitalization as well as test findings (P27). |
| Community transmission | √ Contacts with suspected or confirmed cases with families (P14, P37).√ Relatives and their contact persons if there has been contact with someone suspected or confirmed COVID-19 (P24, P30, P40).√ Investigation with the epidemiological surveillance staff if there are coronavirus cases near patients’ homes (P3, P16, P19). |
| Physical examination | √ Evaluation of skin injuries (P1, P43).√ Presence of intercostal circulation, runny nose, nasal congestion, murmurs, crackling rales, high respiratory rate (P23).√ Careful pulmonary auscultation (P45).√ Peripheral perfusion verification(P51). |
For a better understanding of assumption consolidation, Fig. 2 presents an example of how the process was carried out in order to assemble the information obtained from participants and the literature review, resulting in care assumptions and guidelines for community transmission investigation. The other topics also followed this construction pattern.
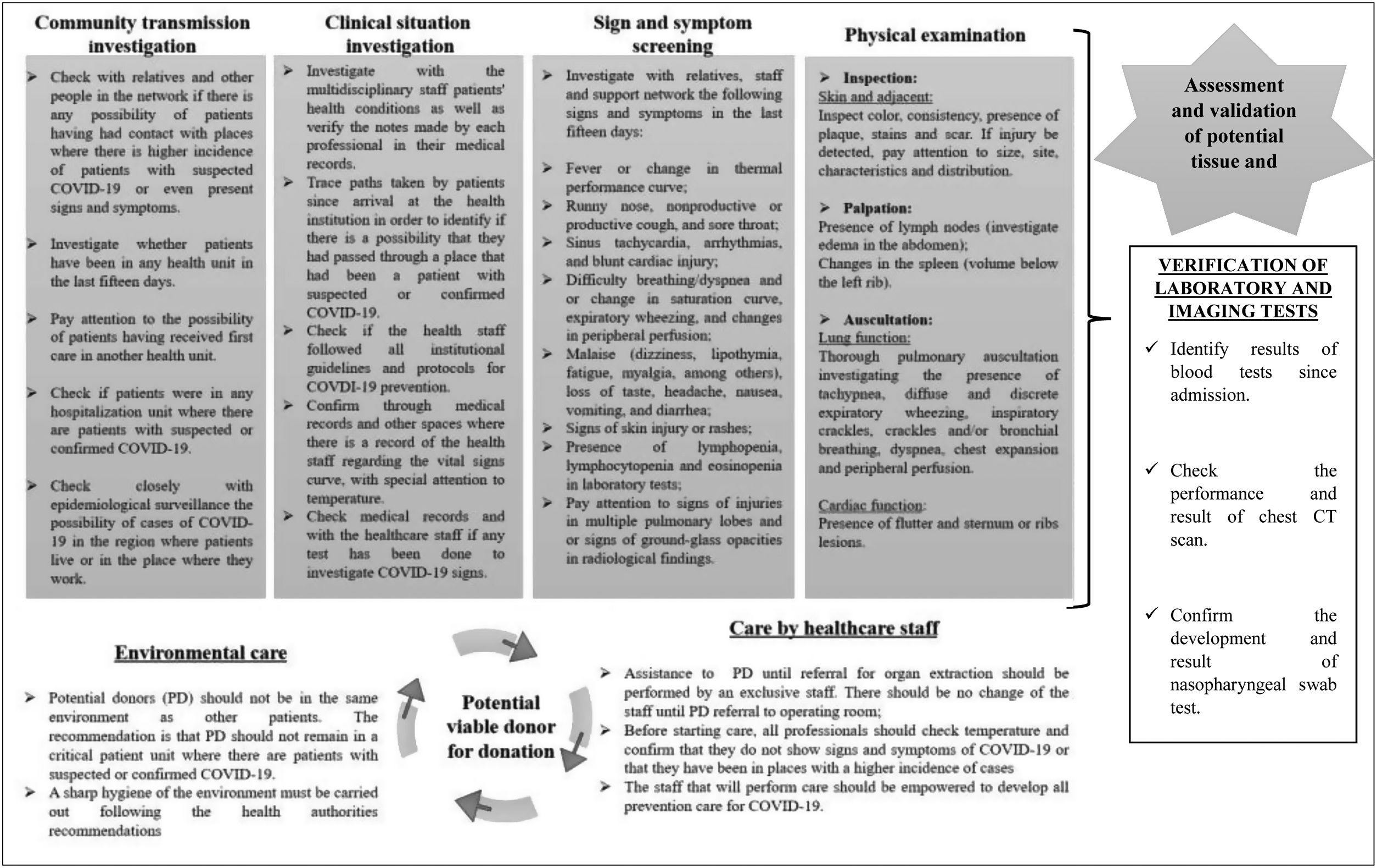
Care assumptions to support and promote safety for registered nurses in decision making about assessment and validation of potential organ and tissue donors in the midst of the COVID-19 pandemic are presented in Fig. 3. Such assumptions will be presented considering the investigation of community transmission, investigation of clinical situations, screening for COVID-19 signs and symptoms, and investigation of changes presented on physical examination, which are formed by 34 care guidelines.
Fig. 3 also shows care assumptions to minimize risk of contamination of potential organ and tissue donors after validation and notification to the State Transplant Center, being composed of different guidelines for environment and staff care.
DiscussionCare assumptions were prepared to guide and support registered nurses during assessment and validation of potential organ and tissue donors. In this perspective, assumptions certainly promote safety, effectiveness and quality in the service offered during the organ and tissue donation process in the midst of the COVID-19 pandemic, in addition to empowering registered nurses in this scenario. Quality and bio-surveillance through the donation stages have been discussed extensively in recent times, in order to improve donation and transplantations by valuing care, safety and quality of life of recipients.34–37
In the first five stages of this study, the findings led to care recommendations regarding carrying out tests and chest CT scans in order to detect SARS-CoV-2, in addition to investigating donor presence in endemic regions and their contact with healthcare professionals during the course of hospitalization. Virtually all studies are for discussion. Regarding the results of phase six of this study, it was possible to notice that there are few professionals trained to work in the organ and tissue donation process in the midst of the COVID-19 pandemic.
Still, Table 2 shows an effort by these professionals to seek guidance and recommendations from health authorities regarding screening and investigation of possible signs of the virus. It is noted that there is a concern to investigate information with patient families and health services, in addition to trying to identify signs and symptoms that may be an alert indicator for COVID-19. There is also a concern for investigative pulmonary aspects.
In this perspective, Fig. 2 reinforces findings in the literature that these professionals, in general, are already following many of the health authorities guidelines to validate potential organ and tissue donors. Table 3 and Fig. 2 present significant contributions from registered nurses who work in practice for developing care assumptions.
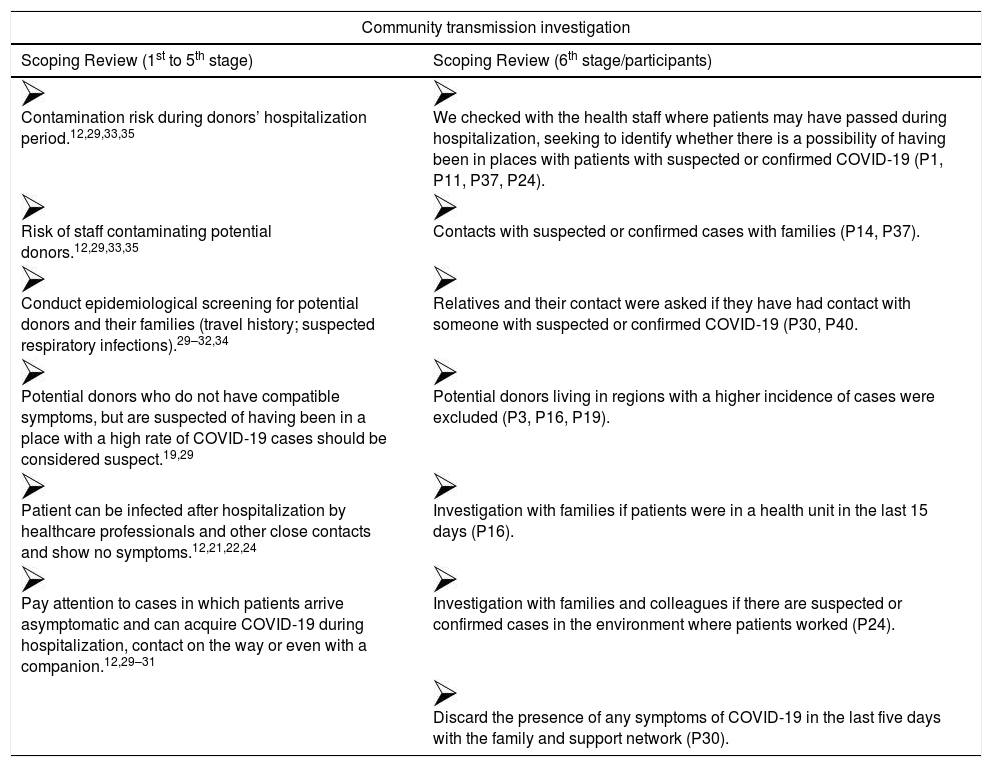
Presentation of the information consolidation obtained in phases 1 to 6 on the community assumption. transmission investigation.
| Community transmission investigation | |
|---|---|
| Scoping Review (1st to 5th stage) | Scoping Review (6th stage/participants) |
| Contamination risk during donors’ hospitalization period.12,29,33,35 | We checked with the health staff where patients may have passed during hospitalization, seeking to identify whether there is a possibility of having been in places with patients with suspected or confirmed COVID-19 (P1, P11, P37, P24). |
| Risk of staff contaminating potential donors.12,29,33,35 | Contacts with suspected or confirmed cases with families (P14, P37). |
| Conduct epidemiological screening for potential donors and their families (travel history; suspected respiratory infections).29–32,34 | Relatives and their contact were asked if they have had contact with someone with suspected or confirmed COVID-19 (P30, P40. |
| Potential donors who do not have compatible symptoms, but are suspected of having been in a place with a high rate of COVID-19 cases should be considered suspect.19,29 | Potential donors living in regions with a higher incidence of cases were excluded (P3, P16, P19). |
| Patient can be infected after hospitalization by healthcare professionals and other close contacts and show no symptoms.12,21,22,24 | Investigation with families if patients were in a health unit in the last 15 days (P16). |
| Pay attention to cases in which patients arrive asymptomatic and can acquire COVID-19 during hospitalization, contact on the way or even with a companion.12,29–31 | Investigation with families and colleagues if there are suspected or confirmed cases in the environment where patients worked (P24). |
| Discard the presence of any symptoms of COVID-19 in the last five days with the family and support network (P30). | |
Source: authors, 2020.
These findings are relevant because registered nurses need to obtain information to assess and validate potential donors, considering that they present many physiological changes due to brain death that are similar to those presented by patients with severe COVID-19. These changes may be related to ineffective cardiac volume; ineffective peripheral tissue perfusion; altered heart rate; ineffective heart rate; altered pulse pressure variation; electrolyte imbalance; important inflammatory process with alteration of leukocytes and dysfunction of endocrine regulation, triggering, in many situations, cardiac arrest and loss of potential donors.35,38,39
Other identified changes are injuries in multiple pulmonary lobes, changes in heart rate, fever, lymphocytopenia and eosinopenia.24,25 It should be noted that there is an incidence of pneumonia associated with mechanical ventilation in approximately 25% of intubated patients.40,41
Considering this, signs and symptoms of COVID-19 may be disguised, as potential donors may develop pneumonia due to intubation. In the scenario of best practices, such care information, combined with the observation of signs and symptoms, directs care management, in addition to minimizing risks and possible errors in the validation of symptoms presented by potential donors.13,14
For registered nurses, such care that is centered on best practices strengthens and supports these professionals during the COVID-19 pandemic. Registered nurses already experience, in their daily work, weaknesses in skills and knowledge to deal with organ and tissue donation.12,42,43 Faced with this new scenario, added to stress and insecurity, the reality becomes more complex, requiring support and care guidelines that can lead this professional through assessment and validation of potential donors.44–46
The COVID-19 pandemic dramatically changed the routine and flow of care in health facilities. Nasopharyngeal swab exam development and tracheal aspiration 24h before organ and tissue donation are recommended. Such procedures should be developed in all patients, both symptomatic and asymptomatic ones, a measure that has been implemented since the beginning of the pandemic.8–10,15,17,46
However, the Brazilian reality, as in other countries, presents a deficit of resources for testing all patients.47 Research shows results of false negative swabs (sensitivity ranging from 70% to 100%) in a significant number of asymptomatic patients. It is important to note that in this study, participants point out that there were few internal adjustments in State Transplant Centers to the reality of COVID-19. Few professionals were trained to validate a potential donor in this context, in addition to only 30.8% of them mentioning the development of epidemiological screening.4,6
Faced with this scenario, over the past six months, health authorities, the scientific community, governmental and non-governmental bodies have been creating regulations, guidelines, technical standards and other tools that can safeguard continuity of transplantations from deceased donors, ensuring a safe and effective process for those patients on the waiting list. Assumptions for best practices developed in this study promote reliability and investigation of risks, uniting the users, environment and healthcare staff in the situation of patients diagnosed with brain death. Therefore, the criteria for viable donors to be notified to the State Transplant Center are adequately taken into consideration.11–13,21
An important step in assessment and validation of potential donors against COVID-19 is community transmission investigation. This moment has become relevant, since it is through questions and targeted investigations that all possibilities will be exhausted regarding potential donors and their contact with infected people.48
Staff must investigate with relatives patients’ daily routines, from work and leisure environment to arrival at the current unit. In other words, questioning whether there were COVID-19 cases in the places where patients worked, whether they had been in places with a high number of contaminated people or frequented places with crowds of people, as well as if they had traveled. It is in these situations when there is a high transmission rate, which is why this step is so necessary.49 Such care guidelines, in best practices, direct risk management to recipients and the community. There is an opportunity to conduct a thorough investigation of the path of potential donors after becoming ill and their contact with people in their community.12,13
Another aspect of the investigation of community transmission concerns hospitalizations or transit in healthcare settings. Due to lack of control in the identification of infected people and the high quantity of this situation, potential donors may have had contact with someone infected in waiting rooms, Health Units, previous hospitalizations and in the current hospitalization itself. For this reason, it is important that registered nurses search exhaustively in the medical records and related documentation, as well as ask the family which healthcare locations the patients have been to. Furthermore, they must investigate other recent hospitalizations seeking to identify whether these units have confirmed coronavirus cases.50,51
Additionally, registered nurses should perform signs-and-symptoms screening of potential donors for COVID-19. For this, a thorough interview with relatives, the support networks and the staff that took care of potential donors is necessary.3 The investigation should seek, from common clinical conditions and upper respiratory tract infection such as fever, malaise, dry cough, diarrhea, nausea, vomiting, headache, to signs and symptoms of more serious diseases such as dyspnea, tachypnea (>30breaths/min), hypoxia (oxygen saturation<90% in room air), pneumonia, lung injuries and acute respiratory distress syndrome.52
A thorough physical examination should be carried out using inspection, palpation, percussion and auscultation, especially of the respiratory and cardiac systems. Another organ system that should be examined in potential donors is the skin, as a study showed the presence of vesicular, urticarial, maculopapular, pseudo-chilblains, pruritus, and herpes injuries in patients with COVID-19. It is noteworthy that interviewing and physical examination are steps of great value for nursing care, since it allows registered nurses to make a complete clinical assessment of potential donors, correct identification of problems, definition of nursing diagnosis, planning and implementation of nursing interventions and monitoring of patient progression. It also enables a professional and autonomous nursing action for decision making.23
Finally, it is worth mentioning that this investigation concerns not only environments with other contaminated patients, but also contaminated healthcare professionals. Asymptomatic infection or even COVID-19 presenting only mild symptoms, in addition to the impossibility of testing all healthcare professionals, can lead to virus transmission by infected professionals to potential donors. It is known that there is a large number of infected healthcare professionals and that this can affect potential donors. This part of the investigation must be the most thorough, and it is necessary to pay attention to the identification of professionals who assisted patients, to question about the presence of symptoms and, most importantly, whether they assisted any other patient with suspected or confirmed COVID-19.51–53
Thus, it is understood that the care assumptions for best practices presented in this study, in addition to highlighting care practices that are capable of minimizing risk of transmission to recipients, support registered nurses’ decision-making and give healthcare staff direction. It should be noted that there is an indication that an isolated staff that does not have contact with possible or confirmed coronavirus cases should be responsible for assistance of potential donors or transplant units; however, even though healthcare professionals understand the pandemic character, there is a possibility that they can acquire the virus while commuting between their residence and the hospital where they work. Therefore, it is important to investigate possible symptoms daily, to ensure proper use of Personal Protective Equipment, and to perform correct hand hygiene and environment care. This demonstrates the need to train professionals to work in this context, in order to reduce the risks of spreading COVID-19 further.48
In the scenario of best practices, all the developed assumptions and care routines provide reliability, investigation and minimization of damages and risks to the donation process, health staff and society.12,13 The European community points out that best practices within organ donation establish standards and guidelines for health institutions and professionals in order to promote service quality.54
ConclusionThe study demonstrated an important review of the literature that supports nurses during the process of evaluating and validating the potential organ and tissue donor during the COVID-19 pandemic.
In addition, demonstrated relevant finds about how registered nurses are doing this in the middle of the pandemic. Thus, this study was able to provide secure information to make decision, alongside the health staff, to validate their patients as potential organ and tissue donors.
Considering such findings, certainly, registered nurses and health staff will be able to follow the pre-established guidelines, leading their practice and empowering these professionals through knowledge to pay attention to the smallest details to be followed and observed before carrying out the validation of organ and tissue donors.
Study limitations: doubts that are still present about the process of infection and development of COVID-19 and the large volume of work demand for registered nurses are shown as limitations. In this context, professional overload and short mastery over the topic makes data collection difficult.
Author's contributionNeide da Silva Knihs: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. João Luis Erbs Pessoa: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. Aline Lima Pestana Magalhães: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. Sibele Maria Schuantes Paim: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. Laísa Fischer Wachholz: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. Elza Lima Silva: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted. Bartira de Aguiar Roza: (1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) Drafting the article or revising it critically for important intellectual content. (3) Final approval of the version to be submitted.
Conflict of interestAll authors declare no conflict of interest.