In Europe, gastric adenocarcinoma (GADC) is commonly regarded as a disease of the elderly. This study aims to assess the proportion, characteristics, and survival of patients diagnosed with GADC under the age of 60.
Materials and methodsThis is a retrospective, multicentric, and analytical study conducted at four tertiary Spanish hospitals. All patients diagnosed with GADC between 2008 and 2015 were included. Demographic, clinical, endoscopic, histologic, and survival data were retrieved. A multivariate analysis was performed to compare GADC in young (age≤60 years) and elderly patients.
ResultsA total of 1374 patients with GADC were included. The mean age was 74 years (SD:11.1); 62.2% were males. There were 177 patients under the age of 60 (12.9%, 95% CI: 11.2–14.8%). GADC was frequently encountered as a metastatic disease in both young and elderly patients (Stage IV: 45.7% and 41%, respectively). In the multivariate analysis, alcohol abuse, ASA functional status I–II, diffuse subtype, neoadjuvant, and palliative therapy were independently associated (P<0.05) with age ≤60 years. No differences were found in 2-year survival (GADC ≤60: 39% vs. 35%, P=0.45). Curative-intent surgery, TNM stage I-II, body mass index<30kg/m2, and better functional status at diagnosis were independent predictors of survival in GADC under the age of 60.
ConclusionsOne out of eight cases of GADC were diagnosed under the age of 60. Metastatic disease was frequent at diagnosis and overall survival was poor regardless of age. Factors associated with localized disease correlated with improved survival in younger patients. Our results underline the need for early diagnosis strategies in our country.
En Europa, el adenocarcinoma gástrico (ADCG) afecta principalmente a pacientes de edad avanzada. Este estudio tiene como objetivo evaluar la proporción, las características y la supervivencia de los pacientes diagnosticados de ADCG menores de 60 años.
Material y métodosEstudio retrospectivo, multicéntrico y analítico realizado en 4 hospitales terciarios españoles. Se incluyeron todos los pacientes diagnosticados con ADCG entre los años 2008-2015. Se recogieron datos demográficos, clínicos, endoscópicos, histológicos y de supervivencia. Se realizó un análisis multivariante para comparar el ADCG en pacientes jóvenes (edad≤60 años) y de edad avanzada.
ResultadosSe incluyeron un total de 1.374 pacientes con ADCG. La edad media fue de 74 años (DE: 11,1), el 62,2% varones. Ciento setenta y siete pacientes tenían menos de 60 años (12,9%, IC 95%: 11,2-14,8%). El ADCG se diagnosticó con frecuencia como enfermedad metastásica en pacientes jóvenes y ancianos (estadio IV: 45,7 y 41%, respectivamente). En el análisis multivariante, el abuso de alcohol, la clase funcional ASA I-II, el subtipo difuso, el tratamiento neoadyuvante y el tratamiento paliativo se asociaron de forma independiente (p<0,05) con una edad ≤60 años. No se encontraron diferencias en la supervivencia a 2 años (ADCG≤60: 39 vs. 35%; p=0,45). La cirugía con intención curativa, el estadio TNM I-II, el índice de masa corporal <30kg/m2 y un mejor estado funcional al diagnóstico fueron factores predictivos independientes de supervivencia en el subgrupo de pacientes menores de 60 años.
ConclusionesUno de cada 8 casos de ADCG se diagnosticaron por debajo de los 60 años. Independientemente de la edad, la presencia de metástasis al diagnóstico fue frecuente y la supervivencia global baja. Los factores asociados a enfermedad localizada se correlacionaron con una mejor supervivencia en pacientes más jóvenes. Nuestros resultados apoyan la necesidad de implementar estrategias de diagnóstico temprano en nuestro país.
Gastric adenocarcinoma (GADC) is the 7th most commonly diagnosed malignancy in Europe and the 5th cause of cancer-specific death.1,2 Annually, around one million people are diagnosed with GADC and 780,000 die of this disease.1 Despite significant improvements in treatment and a steady decline in GADC incidence in the last decades, the condition is often detected at an advanced stage, and recent epidemiological reports indicate that overall 5-year survival remains below 30% in Western countries.1,2
In Europe, GADC is commonly regarded as an entity of the elderly since the majority of patients are over 70 at the time of diagnosis.1 Conversely, some nationwide studies have warned that new cases of GADC among young adults are increasing worldwide.3–6 Available data regarding features and prognostic factors of GADC in younger populations are still limited, especially in Southern Europe. Previous studies have provided controversial results concerning prognosis and GADC characteristics in this selected group. Some reports have observed that survival is significantly lower in younger patients, as a result of later diagnosis and more aggressive behavior with a substantially higher proportion of undifferentiated tumors.7,8 Contrary, other authors have found no direct relation of age with GADC prognosis,9 or even higher survival rates for younger cohorts.10 Besides, distinctive characteristics have been described in young and middle-aged adults with GADC, such as a predominance of females or a higher prevalence of the diffuse-type histology.7,9,11
The present study aimed to determine the proportion, clinicopathological characteristics, and survival of patients diagnosed with GADC under the age of 60 in a Southern European country, and to compare them to those of older patients (> 60 years).
Patients and methodsStudy design and patientsThis was a retrospective, multicentric, and analytical study conducted at four Spanish tertiary hospitals that provide universal public health care assistance to an area with a population of 1.7 million people. All patients diagnosed with histologically proven GADC between January 2008 and December 2015 were included. Gastroesophageal junction (GEJ) Siewert type I tumors were excluded. The study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee for clinical research at the four centers. Informed consent for upper esophagogastroduodenoscopy (EGD) was obtained in all patients. The Ethics Committee accepted exemption of individual informed consent for the inclusion in the study due to its retrospective design.
Study endpointsThe primary endpoint was to evaluate the proportion of patients with GADC who were under the age of 60 years at the time of diagnosis. Secondary objectives included comparing GADC in young (≤60 years) and elderly patients (>60 years) in terms of clinical presentation, histology, oncologic treatment, and survival. Finally, we aimed to detect prognostic factors in young patients.
Data collection and proceduresTwo gastroenterologists independently reviewed paper (hospital) and electronic (hospital and regional primary health care) databases of all subjects at each institution. The following demographic and clinical variables were collected at the time of endoscopic diagnosis: age, sex, tobacco, and active alcohol consumption, family history of GADC, previous gastric surgery (Billroth I, Billroth II, and Roux-en-Y anastomosis), obesity (defined as>30kg/m2 body mass index [BMI]), American Society of Anesthesiologists physical status classification (ASA), symptoms (dysphagia, hematemesis, melena, persistent vomiting, and constitutional syndrome were considered alarm symptoms), proton pump inhibitors (PPI) use, and H. pylori infection.
Gastroscopes used for examination were GIF-Q165, GIF-H180, GIF-H190 (Olympus® Optical, Tokyo, Japan), EG-290KP, EG-294 KP, EG-27I10, EG-29I10, EG-1690K, EG-3490K, EG-2790K (Pentax®, Tokyo, Japan), and EG-530FP, EG-250PES (Fujifilm®, Japan). Endoscopic variables included: date, size of the lesion as assessed by the endoscopist (millimeters), primary location (GEJ, fundus, gastric body, incisura, antrum or anastomosis), and tumor morphology (depressed, flat, sessile or mass-like).
Histologic subtype (intestinal, diffuse or mixed) and grade of differentiation (undifferentiated-poorly or moderately-well differentiated) were retrieved from pathology reports.
Tumor Stage was determined as per the American Joint Committee on Cancer cTNM system 7th edition.12 Oncologic treatment was recorded as binary outcomes: neoadjuvant chemotherapy, curative-intent surgery, adjuvant, and palliative therapy (chemo or radiotherapy). Survival status was established using the date of GADC diagnosis and the time of death. Survivors were right-censored at the date of their last available medical visit (in-hospital or primary health care).
Statistical analysisMean and standard deviation (SD) were calculated for continuous variables and frequency counts and percentages for categorical data. Normality was tested via the Shapiro–Wilk test and plots. Ninety-five percent confidence intervals (CI) for proportions were calculated by the Wilson method. Univariate analysis was performed using the (t-test) for continuous data and the Chi-squared or the Fisher's exact tests for categorical data. Multivariate analysis intended to detect factors associated with GADC≤60 years was performed by binomial unconditional logistic regression. One-year and 2-year overall survival rates were calculated by the Kaplan–Meier method and compared with an unadjusted log-rank test. Cox proportional-hazards regression models were used to: (1) assess the impact of age (≤ 60 or >60 years) on overall survival after adjusting by main confounders (stage, functional status, and treatment); and (2) detect independent predictors of survival in young patients. Proportional hazard assumption was tested through Schoenfeld residuals analysis and time-dependent covariates.
Variables with P values<0.1 in the univariate analysis were considered for multivariate regression. The method of estimating all possible equations was used to create the multivariate predictive models. Hierarchical models with the highest goodness-of-fit and discriminative properties were selected. Multiple imputation by chained equations was used for missing values in the inference analysis. Twenty imputation datasets were created. Missing values are outlined in Table 1 to enhance the transparency of the analysis.
Baseline characteristics stratified by age and univariate analysis.
| Patients≤60 years (n=177) | Patients>60 years (n=1197) | Univariate analysis P values | |
|---|---|---|---|
| Age years, mean (SD) | 52.7 (6.1) | 77.2 (7.7) | |
| Sex male, n (%) | 113 (63.8%) | 742 (62%) | 0.635 |
| ASA, n (%) | |||
| I | 49 (27.8%) | 96 (8.1%) | |
| II | 79 (44.9%) | 381 (32.3%) | |
| III | 48 (27.3%) | 656 (55.6%) | |
| IV | 0 (0%) | 48 (4.1%) | |
| Missing | 1 | 16 | <0.001 |
| Family history of GADC | |||
| Yes | 16 (9.3%) | 75 (7%) | |
| No | 156 (90.7%) | 1002 (93%) | |
| Missing | 5 | 120 | 0.24 |
| Previous gastric surgery | |||
| No | 171 (98.3%) | 1113 (94.4%) | |
| Billroth I | 2 (1.2%) | 21 (1.8%) | |
| Billroth II | 1 (0.6%) | 34 (2.9%) | |
| Roux-en-Y | 0 | 11 (0.9%) | |
| Missing | 3 | 18 | 0.13 |
| H. pylori infection | |||
| Yes | 112 (65.1%) | 713 (63%) | |
| No | 60 (34.9%) | 418 (37%) | |
| Missing | 5 | 66 | 0.55 |
| Smoking | |||
| Yes | 103 (58.2%) | 493 (41.8%) | |
| No | 74 (41.8%) | 685 (58.2%) | |
| Missing | 0 | 19 | <0.001 |
| Alcohol | |||
| Yes | 63 (35.6%) | 237 (20.2%) | |
| No | 114 (64.4%) | 942 (79.9%) | |
| Missing | 0 | 18 | <0.001 |
| Obesity | |||
| Yes | 19 (10.7%) | 189 (15.9%) | |
| No | 158 (89.3%) | 998 (84.1%) | |
| Missing | 0 | 10 | 0.069 |
| PPI use | |||
| Yes | 72 (40.7%) | 584 (48.8%) | |
| No | 105 (59.3%) | 613 (51.2%) | 0.047 |
| Alarm symptoms | |||
| Dysphagia | 19 (10.7%) | 110 (9.2%) | |
| Constitutional syndrome | 63 (35.6%) | 483 (40.5%) | |
| Hematemesis | 12 (6.8%) | 65 (5.5%) | |
| Melena | 12 (6.8%) | 126 (10.6%) | |
| Persisting vomiting | 21 (11.9%) | 153 (12.8%) | |
| None | 49 (27.7%) | 255 (21.4%) | |
| Missing | 1 | 5 | 0.19 |
| Dyspepsia without alarm symptoms | |||
| Yes | 35 (19.8%) | 206 (17.2%) | |
| No | 142 (80.2%) | 991 (82.8%) | 0.4 |
| Primary location | |||
| GEJ | 22 (12.4%) | 92 (7.8%) | |
| Fundus | 20 (11.3%) | 104 (8.8%) | |
| Body | 57 (32.2%) | 415 (35%) | |
| Incisura | 25 (14.1%) | 92 (7.8%) | |
| Antrum | 49 (27.7%) | 455 (38.3%) | |
| Anastomosis | 4 (2.3%) | 29 (2.4%) | |
| Missing | 0 | 10 | 0.003 |
| Size at endoscopy (mm) | 38 | 40 | 0.18 |
| Endoscopic morphology | |||
| Flat-depressed | 58 (32.8%) | 351 (29.3%) | |
| Sessile-Mass | 119 (70.7%) | 846 (70.7%) | 0.4 |
| Histology subtype | |||
| Intestinal | 75 (42.9%) | 689 (57.7%) | |
| Diffuse | 76 (43.4%) | 421 (35.2%) | |
| Mixed | 24 (13.7%) | 84 (7.1%) | |
| Missing | 2 | 3 | <0.001 |
| Grade of differentiation | |||
| Well/moderately | 67 (43.5%) | 597 (55.2%) | |
| Poorly/undifferentiated | 87 (56.5%) | 485 (44.8%) | |
| Missing | 23 | 115 | 0.006 |
| Stage | |||
| I | 16 (9.1%) | 166 (14.2%) | |
| II | 29 (16.6%) | 187 (16%) | |
| III | 50 (28.6%) | 335 (28.7%) | |
| IV | 80 (45.7%) | 479 (41%) | |
| Missing | 2 | 30 | 0.26 |
| Curative-intent surgery | |||
| Yes | 90 (51.1%) | 563 (47%) | |
| No | 87 (48.9%) | 634 (53%) | 0.3 |
| Neoadjuvant chemotherapy | |||
| Yes | 35 (20%) | 88 (7.5%) | |
| No | 140 (80%) | 1081 (92.5%) | |
| Missing | 2 | 28 | <0.001 |
| Adjuvant chemotherapy | |||
| Yes | 63 (36%) | 177 (15.2%) | |
| No | 112 (64%) | 990 (84.8%) | |
| Missing | 2 | 30 | <0.001 |
| Palliative radiotherapy | |||
| Yes | 27 (15.3%) | 99 (8.3%) | |
| No | 149 (84.7%) | 1098 (91.7%) | |
| Missing | 1 | 11 | 0.002 |
| Palliative chemotherapy | |||
| Yes | 55 (31.2%) | 213 (18%) | |
| No | 121 (68.8%) | 973 (82%) | |
| Missing | 1 | 11 | <0.001 |
SD: standard deviation; GADC: gastric adenocarcinoma; ASA: American Society of Anesthesiologist scale; PPI: proton pump inhibitor; GEJ: Gastroesophageal junction. Figures in bold indicate significance.
All tests were two-tailed and P values<0.05 were considered significant. Analyses were performed at the promoting institution (Hospital Universitario Ramón y Cajal, Madrid) using STATA software version 14.1 (StataCorp. Texas, USA).
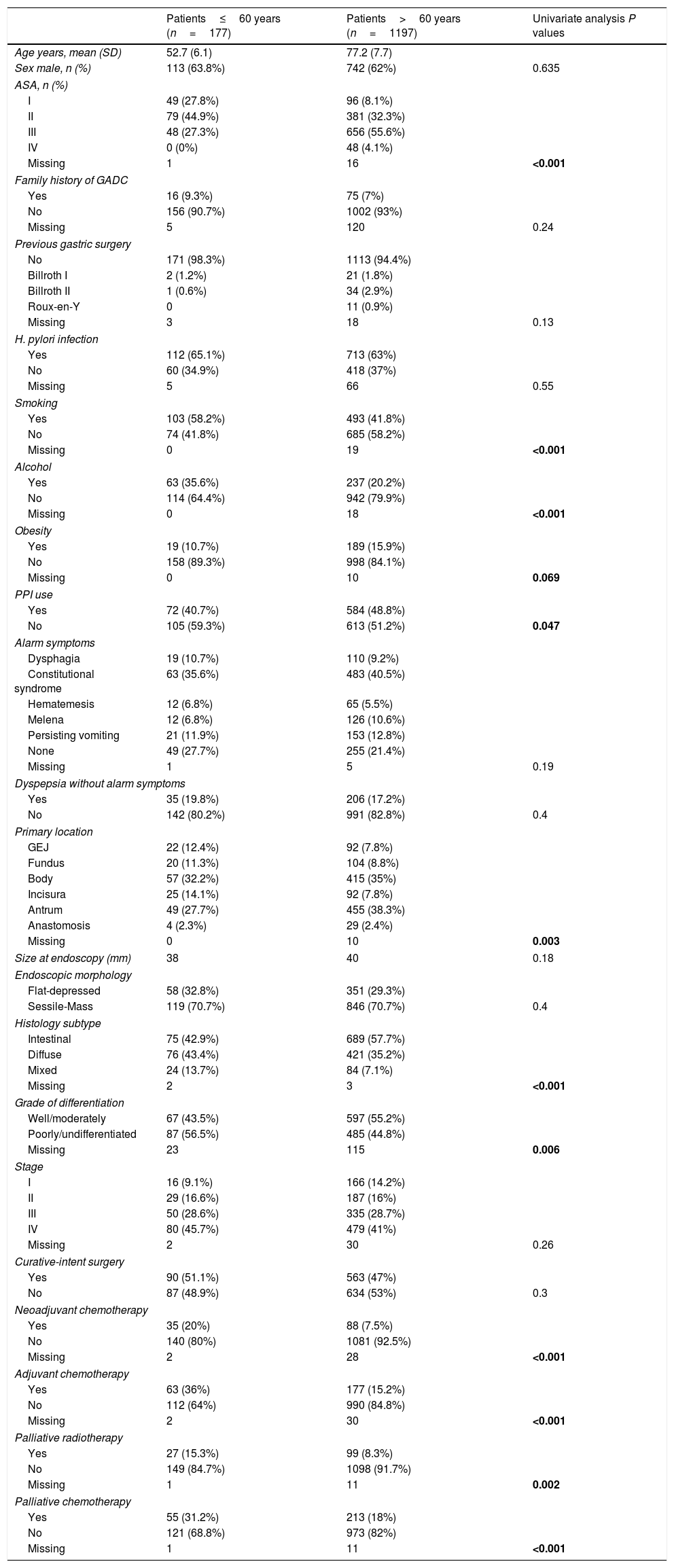
ResultsA total of 1374 patients with GADC were included. The mean age was 74 years (SD: 11.1) and 62.2% were males. One hundred and seventy-seven cases of GADC were diagnosed under the age of 60 (12.9%, 95% CI=11.2–14.8%), 121 (8.8%) between 50 and 60 years, 45 (3.2%) aged 40–50 years, and 11 (0.8%) patients<40 years. Baseline characteristics stratified by age (≤60 and >60 years) are presented in Table 1.
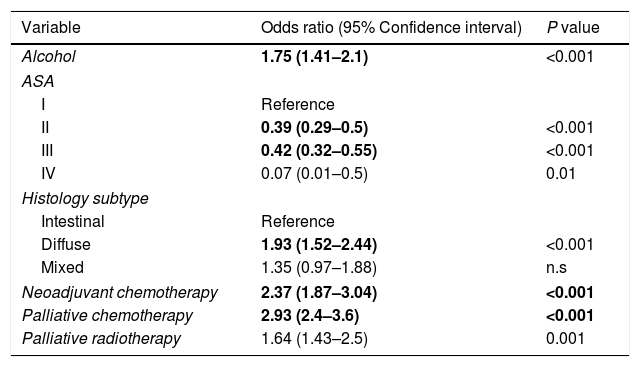
In the univariate analysis, the followings variables were associated with GADC≤60 years (P<0.05): current alcohol consumption, smoking, the absence of PPI, ASA I–II, diffuse subtype, poorly undifferentiated histology, neoadjuvant and adjuvant chemotherapy, and palliative therapy. The gastric body (32.2%) was the most common location for GADC≤60 years and the antrum (38.3%) for elderly patients. Current alcohol consumption, ASA I–II, diffuse histology, neoadjuvant chemotherapy, and palliative therapy remained significantly associated with an age≤60 years in the multivariate logistic regression (Table 2).
Factors associated with gastric cancer under the age of 60 years in the multivariate analysis.
| Variable | Odds ratio (95% Confidence interval) | P value |
|---|---|---|
| Alcohol | 1.75 (1.41–2.1) | <0.001 |
| ASA | ||
| I | Reference | |
| II | 0.39 (0.29–0.5) | <0.001 |
| III | 0.42 (0.32–0.55) | <0.001 |
| IV | 0.07 (0.01–0.5) | 0.01 |
| Histology subtype | ||
| Intestinal | Reference | |
| Diffuse | 1.93 (1.52–2.44) | <0.001 |
| Mixed | 1.35 (0.97–1.88) | n.s |
| Neoadjuvant chemotherapy | 2.37 (1.87–3.04) | <0.001 |
| Palliative chemotherapy | 2.93 (2.4–3.6) | <0.001 |
| Palliative radiotherapy | 1.64 (1.43–2.5) | 0.001 |
ASA: American Society of Anesthesiologist scale; n.s.: nonsignificant. Figures in bold indicate significance.
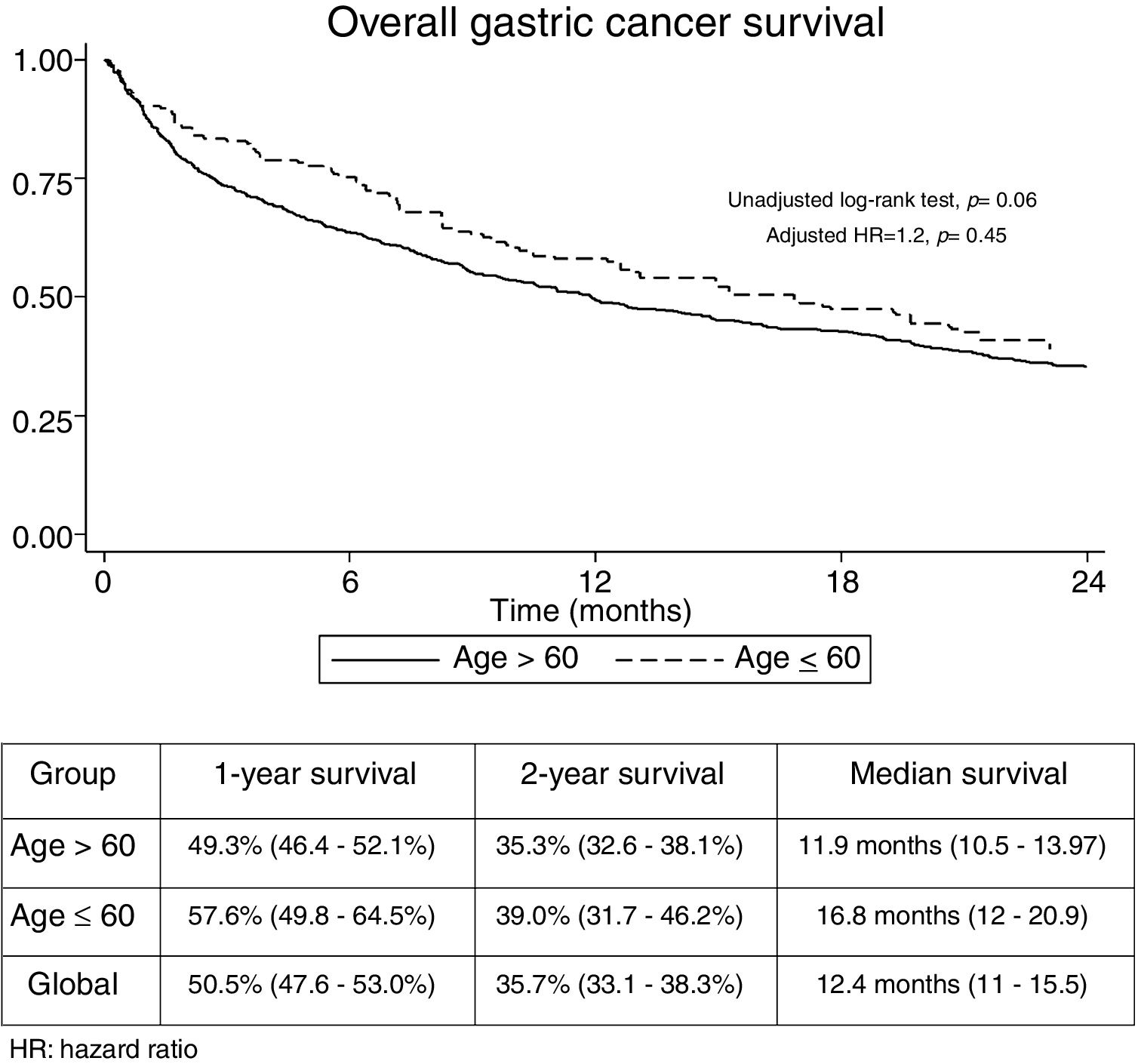
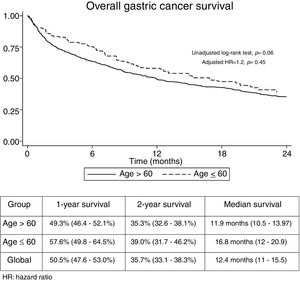
The median follow-up time was 21.3 months (Interquartile range: 6.2–29.3). No significant differences were found regarding overall 1-year (GADC≤60: 57.6% vs. 49.3%) and 2-year unadjusted survival between both groups (GADC≤60: 39% vs. 35.3%; log-rank P value=0.06) (Fig. 1). Age≤60 years (Hazard ratio (HR)=1.2; 95% CI: 0.73–1.96, P=0.45) was not associated with survival after adjusting by TNM stage, functional status, and treatment received.
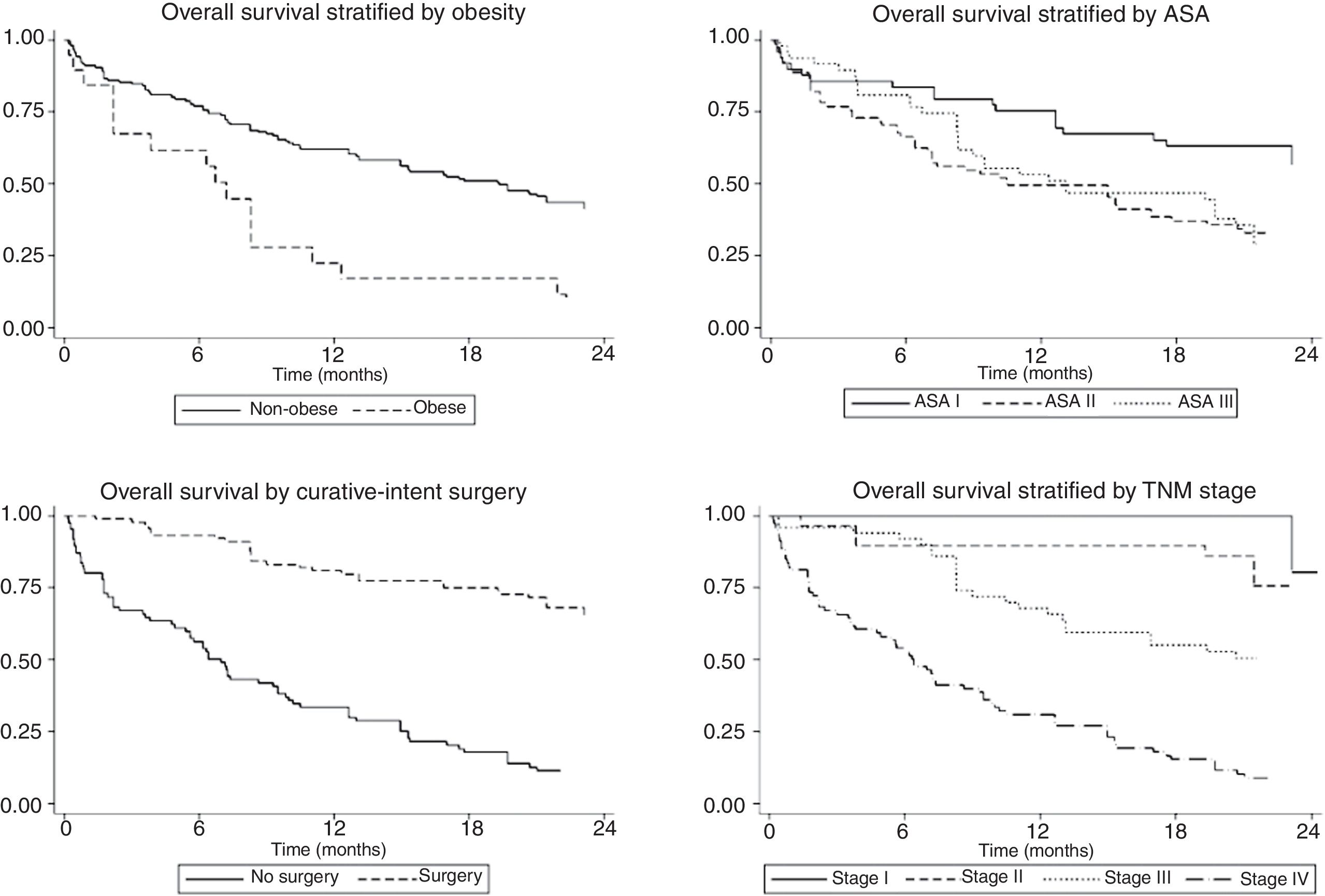
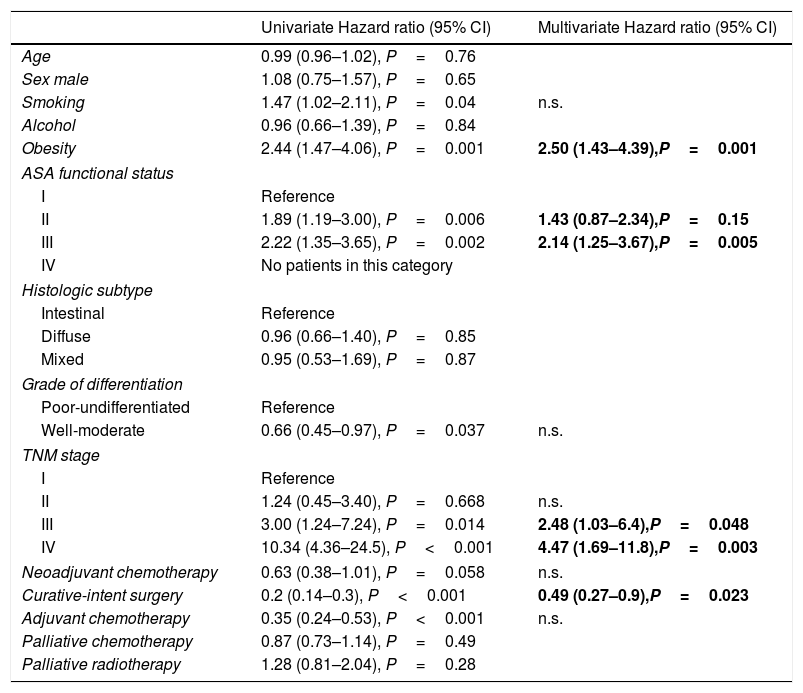
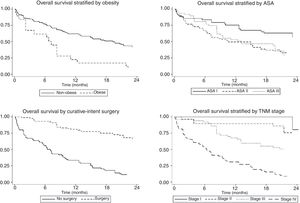
The univariate and multivariate analysis of prognostic factors in patients≤60 years is summarized in Table 3. Obesity (HR=2.50, P=0.001), ASA stage III (HR=2.14, P=0.005), tumor stage III (HR=2.48, P=0.048), and IV (HR=4.47, P=0.003) were associated with worse survival. Curative-intent surgery (HR=0.49, P=0.023) was a significant predictor of improved survival (Fig. 2).
Prognostic factors in patients under the age of 60 years (n=177).
| Univariate Hazard ratio (95% CI) | Multivariate Hazard ratio (95% CI) | |
|---|---|---|
| Age | 0.99 (0.96–1.02), P=0.76 | |
| Sex male | 1.08 (0.75–1.57), P=0.65 | |
| Smoking | 1.47 (1.02–2.11), P=0.04 | n.s. |
| Alcohol | 0.96 (0.66–1.39), P=0.84 | |
| Obesity | 2.44 (1.47–4.06), P=0.001 | 2.50 (1.43–4.39),P=0.001 |
| ASA functional status | ||
| I | Reference | |
| II | 1.89 (1.19–3.00), P=0.006 | 1.43 (0.87–2.34),P=0.15 |
| III | 2.22 (1.35–3.65), P=0.002 | 2.14 (1.25–3.67),P=0.005 |
| IV | No patients in this category | |
| Histologic subtype | ||
| Intestinal | Reference | |
| Diffuse | 0.96 (0.66–1.40), P=0.85 | |
| Mixed | 0.95 (0.53–1.69), P=0.87 | |
| Grade of differentiation | ||
| Poor-undifferentiated | Reference | |
| Well-moderate | 0.66 (0.45–0.97), P=0.037 | n.s. |
| TNM stage | ||
| I | Reference | |
| II | 1.24 (0.45–3.40), P=0.668 | n.s. |
| III | 3.00 (1.24–7.24), P=0.014 | 2.48 (1.03–6.4),P=0.048 |
| IV | 10.34 (4.36–24.5), P<0.001 | 4.47 (1.69–11.8),P=0.003 |
| Neoadjuvant chemotherapy | 0.63 (0.38–1.01), P=0.058 | n.s. |
| Curative-intent surgery | 0.2 (0.14–0.3), P<0.001 | 0.49 (0.27–0.9),P=0.023 |
| Adjuvant chemotherapy | 0.35 (0.24–0.53), P<0.001 | n.s. |
| Palliative chemotherapy | 0.87 (0.73–1.14), P=0.49 | |
| Palliative radiotherapy | 1.28 (0.81–2.04), P=0.28 | |
CI: confidence interval; n.s: nonsignificant. Figures in bold indicate significance.
This study represents an eight-year cohort from four Spanish tertiary hospitals located in an area with medium-high incidence of GADC within our country.13 Our main findings are that the proportion of cases≤60 years was significant (12.9%) and that overall survival was discouraging regardless of age.
The cutoff age for addressing GADC in young populations varies between authors and range from 30 to 70 years.9,10,14,15 We divided patients in ≤60 and >60 years in line with recent reports, the new age subsection-standard of the United Nations World Health Organization, and to test the controversial statement of the current clinical American guidelines for the management of dyspepsia suggesting that GADC is a rarity under this age in Western settings.16–18 The prevalence of younger patients in this study is consistent with previous reports, but some authors have found that up to 45% of GADC may appear during the first six decades of life.7,9–11,15,16,18
According to the available literature, GADC in young populations exhibit a different biological behavior, management and clinicopathological features.9,14,15,19 As expected, younger patients were more likely to receive oncologic treatment, what could be related to better functional status and a more conservative treatment scheme in the elderly. The incidence of GADC in men is almost double that of women,1 but female gender has been reported to be more frequent in younger patients.9,11 This could be partially explained by the hypothesis that estrogens are promoters of GADC carcinogenesis.20 However, this association has been recently questioned, and our results do not support a substantial gender difference.19
We found that undifferentiated and diffuse histology was associated with GADC≤60 years,9,11,14,15 although only the later remained significant in the multivariate analysis. A possible explanation is that the sequence intestinal metaplasia to intestinal-type adenocarcinoma needs several decades to occur.7 No significant differences were detected in terms of H. pylori infection status or clinical presentation. Around 25% of the patients in both age groups lacked alarm symptoms, reinforcing the idea that alarm symptoms are nor sensitive neither specific markers for upper gastrointestinal malignancy.21,22
Concerning tumor location, we found that the gastric body was associated with younger age in the univariate analysis, while the antrum was the most common area for the older counterparts. Only one Japanese study has suggested that GADC at an early age may be more commonly encountered in the middle third of the stomach, but most authors have not detected a consistent association between GADC topography and age.7,14,19,23
Prognostic factors in GADC have been extensively discussed in the literature and this was not the primary aim of our research. Nonetheless, data for the subgroup of younger patients remain inconclusive. For this reason, we decided to perform a stratified analysis focusing on GADC≤60 years. Our survival analysis revealed concerning results that deserve further consideration. First, nearly half of the patients were diagnosed with distant metastasis and less than 30% were at stage I or II. In Europe, it is estimated that only 10–15% of the cases are detected as early cancers, and late diagnosis is the rule rather than an exception.24 Epidemiological studies from Japan and South Korea, where screening population has been extensively implemented, reveal that more than 60–70% of the cases are encountered at local or regional stage.23–26 Second, overall 2-year survival was poor and clearly below Eastern standards where 5-year survival is around 70%.27,28 Third, age is a well-known prognostic factor for several neoplasms, but its impact on GADC survival is still debated. Older studies suggested that younger age was associated with worse outcomes, mainly due to advanced disease at diagnosis and more aggressive behavior.9,23 Others obtained similar results to ours and did not find any relationship.7,9 On the opposite side, some population-database studies indicate that age may be inversely correlated with survival regardless of treatment or stage.10,16,18,29 Even though younger patients were more likely to receive chemotherapy, 2-year survival did not differ significantly. This could be explained by the limited benefit of chemotherapy in the palliative setting where most clinical trials conducted before 2015 provided a survival benefit below 3 months.30 Finally, early-stage disease, BMI<30kg/m2, better functional status, and curative-intent surgery were independent predictors of survival of GADC≤60 years. Indeed, 2-year survival for stage I-II was above 75%, highlighting that only those with localized disease hold the opportunity to obtain optimal oncologic outcomes. Altogether emphasize the urgent need for early diagnosis strategies in Western countries where screening programs have been classically discarded for epidemiological and financial reasons. Interestingly, Areia et al. have recently reported that GADC screening can be cost-effective if combined with screening colonoscopy in an intermediate-risk European setting.31
Our research has some limitations that should be mentioned. Recall bias or selective reporting is a possibility due to the retrospective design of the study. We were not able to collect reliable information regarding genetic mutations (e.g. HER2, GDH1 MLH1, MSH2, MSH6, PMS2, etc.) or serum oncologic markers because of the high heterogeneity in tissue analysis and patient management. The fact that our population is limited to tertiary centers should be considered when extrapolating our results. It should be noted that the proportion of patients treated with neoadjuvant chemotherapy do not represent our current clinical management. Nowadays, neoadjuvant chemotherapy is common practice in most centers as it has shown to improve overall and recurrence-free survival in several randomized trials and meta-analysis.32 Finally, we provide 2-year survival data since a significant number of patients have not completed a 5-year follow-up and obtain more accurate estimates than 5-year outcomes, where missing data could have a higher impact.
In conclusion, GADC indeed occur under the age of 60 and diffuse histology appears to be more common in this subgroup. Metastatic disease at diagnosis was common and overall 2-year survival was below 40%, without significant differences between older and younger patients. Future prospective studies should focus on the most effective strategies to change the current scenario of late diagnosis and poor outcomes in Western countries.
Author's contributionEnrique Rodríguez and Nerea Hernanz designed the study. The initial protocol was critically appraised by all the authors before the study outset. Enrique Rodríguez performed the statistical analysis and draft the initial version of the manuscript. The remaining authors critically interpreted the data and revised the manuscript for relevant intellectual content. All authors fully approved the final version of the manuscript.
Financial supportThis study did not receive any grant or financial support.
Conflicts of interestThe authors declare that there is no conflict of interest.