Crohn’s disease (CD) is a chronic inflammatory disorder that involves different sites along the length of the gatrointestinal tract. The well known transmural and scarring character of this inflammatory bowel disease can explain the common local complications, such as deep sinus tracts and fistulas, mesenteric abscesses, and obstruction1. Although remarkable advances have occurred in recent years in the diagnosis and therapy of CD, the factors which lead to differences in the clinical evolution of this disorder among diverse patients are still unknown1. The finding of pathophysiologically relevant markers reflecting disease progression would be helpful in order to predict the evolution of CD patients or to optimize the treatment for each individualized patient. Diverse serum mediators have been analyzed in order to propose them as biological markers, but a general agreement does not exist in this field2. Angiogenesis, or the formation of new vessels from preexisting ones, is a natural process that occurs in the organism in association with both healthy and pathologic conditions3. This complex process, which involves endothelial cell proliferation, migration, and adhesion, matrix remodeling, recruitment of pericytes and stabilization of neovessels, is triggered through mechanisms requiring the participation of proinflammatory cytokines and growth factors3,4. Inflammatory mediators can, either directly or indirectly, promote angiogenesis. Angiogenesis, in turn, contributes to pathological inflammation by permitting the recruitment of inflammatory cells; therefore, inflammation and angiogenesis are linked processes5. Neoangiogenesis is tightly regulated by a balance of proand anti-angiogenic factors, which are expressed in response to external stimulatory or inhibitory agents6. Under pathological conditions, an excess of pro-angiogenic factors occurs, leading to an ‘angiogenic switch’, which initiates the pathological formation of new vessels6. In the case of chronic inflammatory diseases, angiogenesis is initiated as a defence mechanism aimed at preventing tissue ischemia caused by inflammatory infiltrates and at facilitating the access of immune cells to the site of injury. However, the newly formed vascular network is structurally and functionally abnormal and may also con-tribute to tissue damage7. Derived from several studies, blocking agents against angiogenic factors have been purposed as potential candidates for diverse chronic inflammatory diseases treatment8. The study of the relationship between vascular development and inflammatory bowel diseases could be helpful to elucidate the pathogenesis of this chronic inflammatory disease7,9. Among the major mediators implicated in the angiogenic process are the members of vascular endothelial growth factor (VEGF) family (VEGF and placental growth factor, PlGF), the angiopoietins (Ang), both Ang-1 and Ang2, and their respective tyrosine-kinase receptors: VEGF receptors (VEGFR1 and VEGFR2) and Ang1 and Ang2 receptor, Tie23,6,7.
Little data exist regarding the role of soluble angiogenic factors in the pathogenesis of CD. Regarding VEGF, previous results are controversial, possibly due to the diverse population of the patients studied and the differences in the laboratory’s techniques used9-13, which are probably not suitable for comparing them. In the case of Ang2 and Tie2, one study has measured their serum levels in patients with active and inactive CD and found significant differences in respect to the healthy controls14. Therefore, as angiopoietins may play a critical role in adult angiogenesis related with the inflammation, they should be further investigated in CD. In this preliminary study, we analyzed whether serum levels of angiogenic factors are increased in patients with CD as compared with healthy controls. We further evaluated whether patients with distinct CD clinical phenotypes express different amounts of angiogenic factors in order to find a possible marker, which could predict the evolution of the EC patients. Thus, serum levels of several angiogenic factors (VEGF, PlGF, and their receptors, as well as the angiopoietins –Ang1 and Ang2–, and the receptor Tie2) were studied in CD patients, which were stratified into inflammatory, fibrostenotic and fistulizing sub-types. Finally, we attempted to elucidate the possible separate or combined role of all these soluble factors on CD.
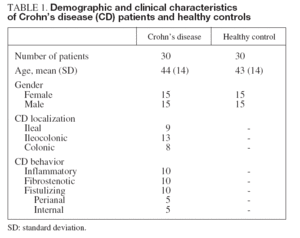
METHODSPatients and controlsThirty patients (50% female, ranging in age from 23 to 72 yrs; mean age, 44 ± 14 yrs) with a diagnosis of CD and Crohn’s Disease Activity Index (CDAI) ¿ 150 were studied. Diagnosis of CD was established by standard clinical, radiological, histological, and endoscopic criteria15. Patients were matched for age and sex with thirty healthy controls (50% female, ranging in age from 25 to 71; mean age, 43 ± 14 yrs). Using the Vienna classification, CD patients were classified according to their clinical phenotypic behavior into 3 equal sub-groups: inflammatory, fibrostenotic and fistulizing. Signed informed consent was obtained from each participant under a protocol approved by the Ethical Committee on Clinical Investigation of our hospital. All patients and controls were Caucasian. Patients receiving biologic therapies, or those presenting with cancer, coronary disorders, malignant hypertension and pregnant women were excluded from this study. Serum levels of standard laboratory parameters including hemoglobin, leukocyte count, platelet count, albumin, erythrocyte sedimentation rate, C-reactive protein, fibrinogen and orosomucoid were measured by routine determination.
Determination of angiogenic soluble factorsSerum levels of VEGF, PlGF, VEGFR1, VEGFR2, Ang1, Ang2 and Tie2 were determined both in CD patients and healthy controls. Peripheral blood samples were allowed to clot for 30 min at –4 ºC temperature before centrifugation for 10 min at 2500-3000 rpm. Serum was stored at –80 ºC. The angiogenic soluble factors levels were measured by enzyme-linked immunosorbent assays (ELISA) (Human Immunoassay Quantikine, R&D Systems, Minneapolis, USA). Their concentrations were determined by the standard curve obtained when using the standards supplied with the kit according to the manufacturer’s instructions.
Statistical analysisFor quantitative variables, mean and standard deviation were calculated. Student T-test was used to carry out the comparison between serum levels of angiogenic factors in CD patients and healthy controls. Statistical analysis in order to evaluate the differences of angiogenic soluble factors concentrations depending on the behavior of the disease was performed by the analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons. The relationship among the serum levels of the diverse angiogenic factors was analyzed by a linear regression model. A value of p < 0.05 was considered statistically significant.
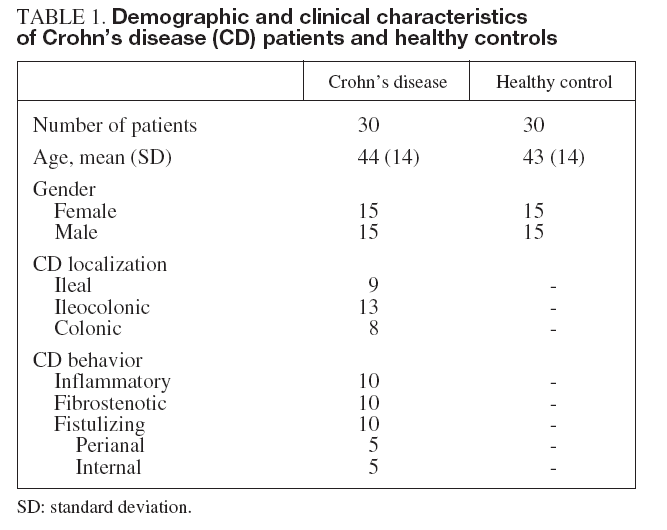
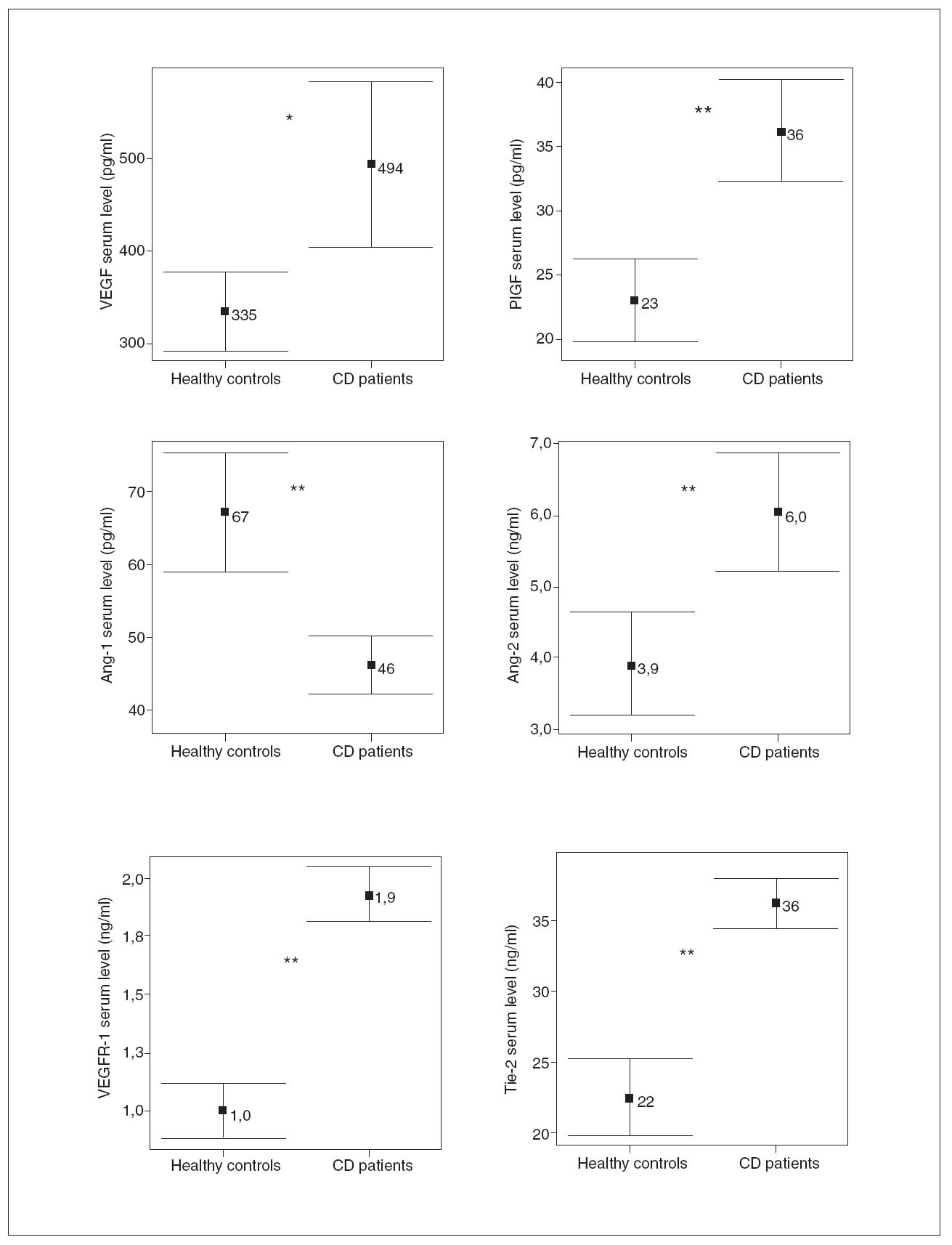
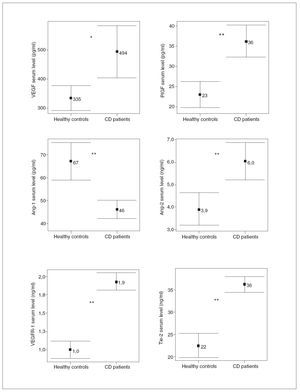
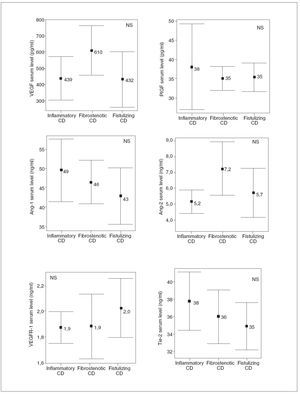
RESULTSDemographic and clinical characteristics of both CD patients and healthy controls are summarized in table I. Of the 30 evaluated patients, 50% were women, 50% smokers, 10% had extraintestinal manifestations and 43% had ileocolonic disease. The mean of the evolution of the disease was 10.2 ± 6 years. With regard to the phenotypic behavior of the disease, 10 patients were included in each sub-group: 10 inflammatory, 10 fibrostenotic and 10 fistulizing. 76.7% of EC patients received maintenance therapy: 20.0% aminosalycilates and 53.3% immunomodulators, including azathioprine, mercaptopurine and methotrexate. In CD patients, most of the soluble angiogenic factors showed significantly higher serum levels than in healthy controls (VEGF: 494 ± 247 vs. 335 ± 118 pg/ml, p < 0.01; PlGF: 36 ± 11 vs. 23 ± 9 pg/ml, p < 0.001; VEGFR1: 1.9 ± 0.3 vs. 1.0 ± 0.3 ng/ml, p < 0.001; Ang2: 6.0 ±2.3 vs. 3.9 ± 2.0 ng/ml, p < 0.001; Tie2: 36 ± 5 vs. 22 ± 7 ng/ml, p < 0.001) (fig. 1). Conversely, lower Ang1 serum levels were observed in CD patients than in healthy controls (46 ± 11 vs. 67 ± 23 ng/ml, p < 0.001) (fig. 1). However, no difference existed in VEGFR2 serum levels between CD patients (10.2 ± 2.0 ng/ml) and healthy controls (10.4 ± 3.2 ng/ml). Results were also compared according to the behavior of CD. In patients with fibrostenotic CD, a tendency towards increased VEGF (610 ± 237 pg/ml) and Ang2 (7.2 ± 2.6 ng/ml) levels compared to those with inflammatory (439 ± 213 pg/ml and 5.2 ± 1.2 ng/ml, respectively) or fistulizing (432 ± 268 pg/ml and 5.7 ± 2.4 ng/ml, respectively) behavior was observed (fig. 2). On the other hand, the patients with inflammatory phenotype showed slightly elevated PlGF (38 ± 18 pg/ml), Ang1 (49 ± 12 ng/ml), and Tie2 (38 ± 5 ng/ml) levels compared with fibroestenotic (35 ± 5 pg/ml, 46 ± 9 ng/ml and 36 ± 5 ng/ml), and fistulizing (35 ± 6 pg/ml, 43 ± 11 ng/ml and 35 ± 4 ng/ml) behavior (fig. 2). However, these trends did not reach statistical significance, probably due to the limited sample size. Noteworthy is the fact that there were statistically significant elevated Ang1 serum levels in CD patients with perianal fistulas in comparison with those with internal fistulas (51 ± 11 vs. 35 ± 3 ng/ml, p < 0.05). Significant differences of the levels of the angiogenic factors were not observed when EC patients were classified according to the fact of presenting stenosis (yes/no) or fistulas (yes/no), independently. When the CD patients were compared according to their disease location pattern, differences did not reach statistical significance. In colonic CD, VEGF (336 ± 151 pg/ml) and Ang2 (5.4 ± 1.7 ng/ml) serum levels were lower than in ileal (590 ± 268 pg/ml; 7.1 ± 2.8 ng/ml, respectively) or ileocolonic CD (524 ± 247 pg/ml; 5.7 ± 2.1 ng/ml, respectively).
TABLE 1. Demographic and clinical characteristics of Crohn’s disease (CD) patients and healthy controls
The EC patients who received maintenance therapy (76.7%) showed an increase of the levels of VEGF and a decrease of the VEGFR2 levels as compared with those who did not take such medication (557 ± 243 vs. 287 ± 113, p < 0.001; 9.8 ± 2.0 vs. 11.3 ± 1.2, p < 0.05, respectively). Additionally, significant altered levels of VEGF and Ang1 in EC patients who took immunomodulators regarding those who did not take azathioprine, mercaptopurine or methotrexate as maintenance therapy were observed (VEGF: 583 ± 236 vs. 391 ± 224, p < 0.05; Ang1: 42 ± 10 vs 51 ± 10, respectively). The correlation between pairs of these angiogenic factors was also studied. VEGF correlated positively with Ang2 (r = 0.42, p = 0.001). VEGFR1, as well as its ligand, PlGF, correlated positively with Ang2 (r = 0.42, p = 0.001; r = 0.30, p = 0.019, respectively) and Tie2 (r = 0.76, p < 0.001; r = 0.53, p < 0.001, respectively). However, they showed negative correlation with Ang1 (r = –0.45, p < 0.001; r = –0.30, p = 0.021, respectively). Correlations were also observed between receptors and its ligands. Thus, VEGFR1 correlated with PlGF (r = 0.58, p < 0.001), and Tie2 with Ang2 (r = 0.30, p = 0.021, positively) and with Ang1 (r = –0.49, p < 0.001, negatively). Moreover, CD patients showed a statistically significant correlation between serum VEGF levels and leukocyte count (r = 0.42, p = 0.03). Finally, serum levels of both VEGFR1 and VEGFR2 were correlated with orosomucoid (r = 0.49, p = 0.021; r = 0.44, p = 0.042, respectively).
DISCUSSIONIn the current preliminary study, CD patients presented a pro-angiogenic profile reflected by elevated serum levels of two angiogenic systems consisting of ligands and their receptors, VEGF-PlGF/VEGFR1 and Ang1-Ang2/ Tie2. This supports the hypothesis that an alteration in the neoangiogenic process participates in the pathogenesis of CD. Most of our knowledge about the VEGF
Fig. 1. Comparison between angiogenic soluble factors in healthy controls versus Crohn’s disease (CD) patients. *p < 0.01. **p < 0.001. Error bars plots are given with means and standard deviations.
Fig. 2. Comparison of the angiogenic soluble factors depending on the behavior of the Crohn’s disease (CD). NS: non-statistically significant differences for all comparison. Error bars plots are given with means and standard deviations.
PlGF/ VEGFR1 system arises from recent oncology studies in which the PlGF has shown to play an important role in the angiogenic switch in pathological angiogenesis by interacting with VEGFR1 and synergizing with VEGF16. In theory, we would expect that the soluble form of the VEGFR1 would function as a «decoy» during the development of blood vessels, and prevent VEGF from binding to its signaling receptors. However, PlGF, which is produced and released in abundant amounts by activated endothelial cells, could displace VEGF from VEGFR1, thereby favoring the fraction of VEGF available to bind to and activate VEGFR217. The same may occur with the extracellular domain of Tie2 that is detectable in serum and its two ligands, Ang1 and Ang2. One of them may displace the other to interact with the binding ligand site of the soluble receptor. In our study, CD patients presented statistically significant higher serum levels of VEGF, PlGF and VEGFR1 than healthy controls. Few reports exist that focus on the differences of angiogenic serum factor levels between CD patients and healthy controls. Our findings are consistent with previous reports, that were carried out in children and young adults with CD11,18, in which an increased number of VEGF and VEGFR1-positive blood vessels in colonic mucosa were seen10. Conversely, other authors observed either no differences between these two groups9,12,19 or even lower serum levels of VEGF in CD patients13,20. In the case of the Ang1-Ang2/Tie2 system, information on its pathophysiological role in CD is even scarcer. There is only one study that has observed elevated Ang2 and Tie2 serum levels in active CD patients compared with healthy controls14 and, to the best of our knowledge, the present study is the first one to examine Ang1 serum levels and the relationships among diverse angiogenic factors in CD patients. Both Tie2 and Ang2 levels are increased in CD patients in remission, allowing the development of new vessels, together with the system VEGF-PlGF/VEGFR1. By contrast, the Ang1 levels in these patients were lower than in healthy controls, suggesting that there is a decoy of the constitutive stabilizing effects of this angiopoietin on new blood vessels. This suggests that an abnormal and pathological angiogenesis in the bowel wall exists, and thereby further studies would be necessary to evaluate if these new vessels are irregularly shaped, tortuous, and lack the normal hierarchical arrangement, as in the case of tumor blood vessels21. These results are consistent with the knowledge that angiogenesis requires blockage of Ang1mediated signaling and hence Ang2 tends to be upregulated by stimulus associated with angiogenesis including VEGF22. Perhaps the most interesting observations in this study are the differences of soluble angiogenic factors when the patients were classified according to CD behavior. However, these differences were not statistically significant, possibly due to the limited number of patients investigated. The fibroestenotic behavior of CD was characterized by elevated VEGF and Ang2 levels, which could favor the formation of new blood vessels, supplying nutrients and oxygen to the new tissue. On the other hand, an in-crease of Tie2 and PlGF levels in the inflammatory pattern, both of them previously related with inflammation6 and activation of monocytes23, was observed. Moreover, the differences in Ang1 serum levels of CD patients with internal or perianal fistulas could reflect the local disturbed equilibrium between the systems of angiopoietins. These findings could be helpful to elucidate the evolution of each CD patient and thereby to adapt the follow-up or the therapeutic strategy. The intake of maintenance therapy, especially immunomodulators, may have a significant influence on the levels of VEGF, VEGFR2 and Ang1 in EC patients. However, in our opinion these results are not conclusive due to the inequality of the group compared. Observed correlations between some pairs of angiogenic factors reflect a possible interplay between these systems of angiogenic mediators. Thus, it could be more appropriate to evaluate several angiogenic mediators rather than only one. Moreover, the obtained correlation among the members of the system Ang1-Ang2/Tie2 reflects, as already described, the necessary balance between resting and activated vascular endothelium5. To conclude, angiogenesis in CD patients appears to play a role in CD reflected by abnormal levels of angiogenic mediators. Moreover, differences seem to be present in patients according to their clinical phenotypic behavior. Wider and prospective studies are necessary to determine if these angiogenic soluble markers could be useful to predict the evolution of patients with CD.
ACKNOWLEDGEMENTSThis study was supported by a grant (to ID Pousa) from Schering Plough and by grants (to JP Gisbert) from Instituto de Salud Carlos III (C03/02 and PI050109).
Correspondence: J.P. Gisbert.
Playa de Mojácar, 29. Urb. Bonanza. 28669 Boadilla del Monte (Madrid). España.
E-mail: gisbert@meditex.es
Recibido el 21-3-2007; aceptado para su publicación el 5-6-2007.