Autoimmune hepatitis induced by drugs represents an increasingly known category of hepatotoxicity due to medication .1,2 This liver disorder is also known as drug induced autoimmune like hepatitis or autoinmune-like drug-induced liver injury. Some of the drugs associated with this pathology include nitrofurantoin, minocycline, diclofenac, anti-TNF-α, α-methyl DOPA and statins .1,2 We present a case report of a patient treated with rosuvastatin who showed liver injury with autoimmune features.
A 47 year-old white man with history of ischemic cardiopathy and hyperlipoproteinemia, with previous normal liver enzyme values, came to our outpatient clinic due to hypertransaminasemia. The patient denied any history of autoinmune disorders, liver diseases, alcohol or herbs use. Eleven months earlier he had started treatment with Rosuvastatin (he had switched from Atorvastatin). He was also receiving aspirin 100mg and Bisoprolol 2.5mg. Ten months after starting treatment with Rosuvastatin revealed a serum alanine aminotransferase (ALT) of 201 U/L (n: 0-41 U/L) and aspartate aminotransferse (AST) of 112 U/L (n: 0-37) (Table 1). The patient showed no symptoms and Rosuvastatin was discontinued. About 2 weeks after stopping therapy with Rosuvastatin, aspartate animotransferase and alanine aminotransferase levels continued to increase (AST 151 U/L, ALT 266 U/L). Serological testing for hepatitis A, B and C were negative, as were testing for Epstein-Barr virus and cytomegalovirus. Antinuclear antibodies were present (160); test for antismooth muscle antibodies, antimitochondrials and anti-LKM were negative and IgG was normal. Liver ultrasound examination was normal too. A study for leucocyte antigen revealed HLA-DRB1 *11:04 and *15:01; DR3 and DR4 were absent. Autoimmune Hepatitis (AIH) international score was 11(probable), CIOMS/RUCAM Score was 5 (possible) and AIH-drug induced liver injury (DILI) was presumed. A month after discontinuing the drug, the ALT-AST values started to show a slow improvement. Anti-smooth muscle antibodies were present (title 1:80) as well as antinuclear antibodies (1:80). ALT and AST values continued decreasing and 2 months after stopping Rosuvastatin intake auto-antibodies became negative. Finally, during the following 2 weeks (3 months after discontinuing Rosuvastatin) AST-ALT levels dropped back to normal and stayed that way for the next 12 months’ follow up, when the patient was discharged. Our patient remained asymptomatic during the whole process and no corticotherapy was used.
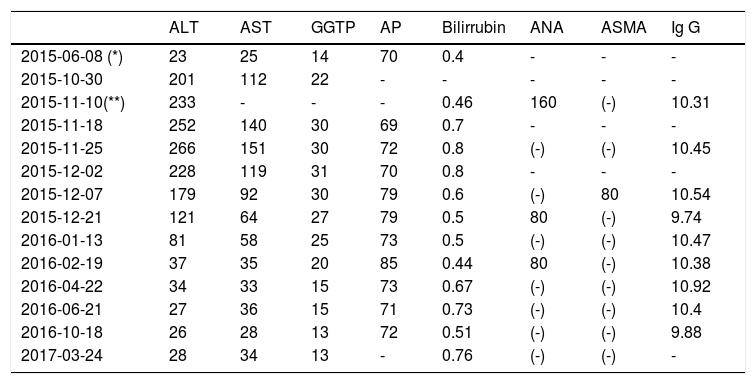
Main laboratory data (biochemical evolution) from the case report.
| ALT | AST | GGTP | AP | Bilirrubin | ANA | ASMA | Ig G | |
|---|---|---|---|---|---|---|---|---|
| 2015-06-08 (*) | 23 | 25 | 14 | 70 | 0.4 | - | - | - |
| 2015-10-30 | 201 | 112 | 22 | - | - | - | - | - |
| 2015-11-10(**) | 233 | - | - | - | 0.46 | 160 | (-) | 10.31 |
| 2015-11-18 | 252 | 140 | 30 | 69 | 0.7 | - | - | - |
| 2015-11-25 | 266 | 151 | 30 | 72 | 0.8 | (-) | (-) | 10.45 |
| 2015-12-02 | 228 | 119 | 31 | 70 | 0.8 | - | - | - |
| 2015-12-07 | 179 | 92 | 30 | 79 | 0.6 | (-) | 80 | 10.54 |
| 2015-12-21 | 121 | 64 | 27 | 79 | 0.5 | 80 | (-) | 9.74 |
| 2016-01-13 | 81 | 58 | 25 | 73 | 0.5 | (-) | (-) | 10.47 |
| 2016-02-19 | 37 | 35 | 20 | 85 | 0.44 | 80 | (-) | 10.38 |
| 2016-04-22 | 34 | 33 | 15 | 73 | 0.67 | (-) | (-) | 10.92 |
| 2016-06-21 | 27 | 36 | 15 | 71 | 0.73 | (-) | (-) | 10.4 |
| 2016-10-18 | 26 | 28 | 13 | 72 | 0.51 | (-) | (-) | 9.88 |
| 2017-03-24 | 28 | 34 | 13 | - | 0.76 | (-) | (-) | - |
Baseline (*) and (**) one day after drug-cessation laboratory. ALT (N: 0-41 U/L)-alanine aminotransferase; AST (N: 0-37 U/L)-aspartate aminotransferase; GGTP (N: 10-71 U/L)-gammaglutamil-transferase; AP (N: 40-129)-alkaline phosphatase; bilirrubin (N: 0-1.1mg/dL); ANA: antinuclear antibodies; ASMA: anti smooth muscle antibodies; Immunoglobulin G (N: 7-16g/L).
Several cases of autoimmune hepatitis associated with statins have been reported.3–7 Two different scenarios may be distinguished: Drug induced (DI)-AIH, a selfperpetuating disease, and immune-mediated (IM)-DILI, an acute or chronic process that disappears after drug withdrawal8 (which we presume our patient's case to be).
Usually symptoms of acute liver injury occur within the first months after starting therapy but a longer latency period is possible1 (as shown in our patient). A spontaneous remission after drug cessation is expected in IM-DILI but sometimes hepatocellular or mixed type liver damage may appear.8 If so immunosuppressive (IS) treatment is mandatory.8 Response to corticoesteroids therapy is usually outstanding with complete remission and no relapse even after discontinuing immunosuppressive therapy.1,8 Therefore IS treatment is not forever and this distinguishes IM-DILI from idiopathic autoimmune hepatitis and DI-AIH.8
Statins are responsible of an important number of cases with DI-AIH (IM-DILI) and DILI with positive autoantibodies3,8. It is very difficult to differentiate between this two clinical scenarios8,9. The weaknessess of our case are the absence of liver biopsy, the normal Ig G values, and the absence of typical HLA-DR alleles. None of them is mandatory for the diagnosis of DI-AIH, although they are very helpful6,9,10. We have not performed a liver biopsy because of the good evolution and the mild clinical features of the patient. Higher Ig G values are associated with DI-AIH, but normal values may be present in these patients6,10. The presence of positive autoantibodies (ANA, ASMA) and the loss of ANA positivity after cessation of the drug are more likely to be associated with DI-AIH. The latency period of more than 2 months, generally longer than DILI patients, and hepatocellular liver injury pattern, are tipically associated with this disease. Recovery time is expected to be longer for DI-AIH than for DILI (8-10 weeks vs 5-7 weeks)6,10. All of these criteria were fulfilled by our patient. Morover, the transaminases values do not decrease more than 50% in the first month after drug cessation, and, although CIOMS score was possible for DILI, AIH international score revealed probable for AIH. Therefore, we think that this case report is a very probable IM-DILI due to statins.
Conflict of interestNo concflicts of interest